Market Overview:
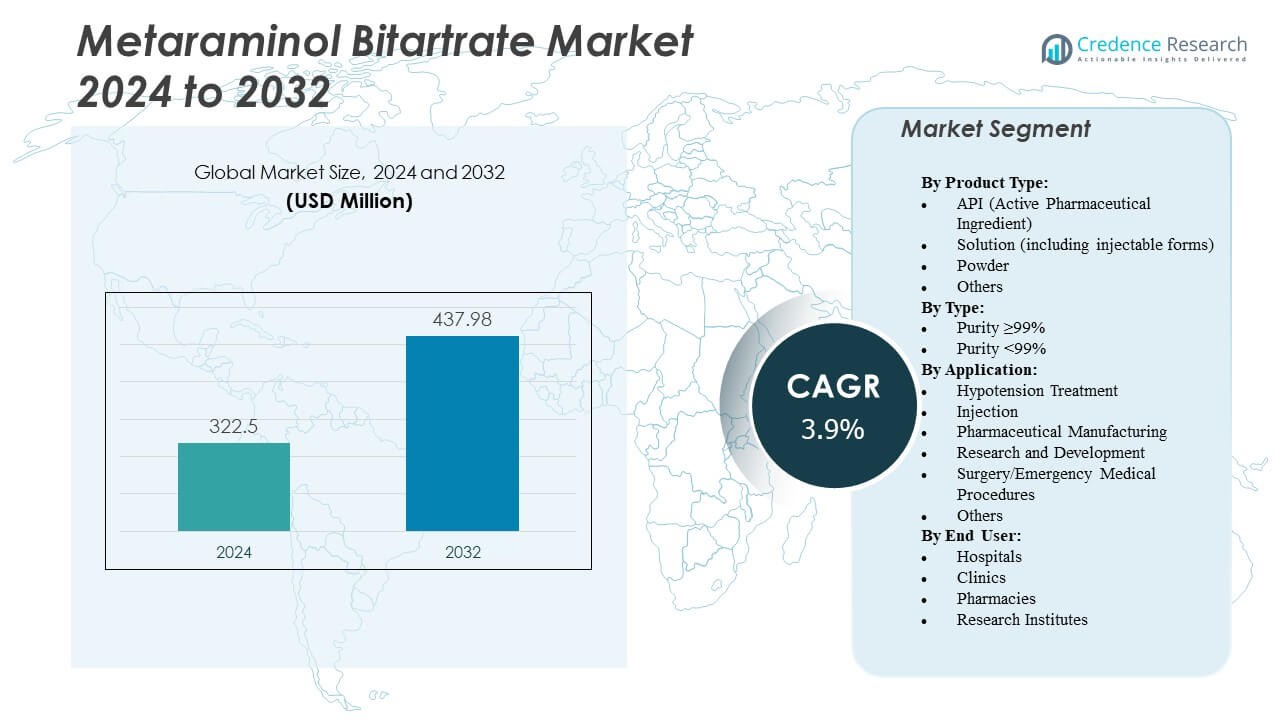
The Metaraminol Bitartrate Market is projected to grow from USD 322.5 million in 2024 to an estimated USD 437.98 million by 2032, with a compound annual growth rate (CAGR) of 3.9% from 2024 to 2032.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Metaraminol Bitartrate Market Size 2024 |
USD 322.5 Million |
| Metaraminol Bitartrate Market, CAGR |
3.9% |
| Metaraminol Bitartrate Market Size 2032 |
USD 437.98 Million |
Market growth is driven by the rising prevalence of cardiovascular diseases and hypotension cases requiring vasopressor therapy. The drug’s increasing use in hospitals and surgical centers for maintaining blood pressure stability during anesthesia and critical care has strengthened demand. Expanding healthcare infrastructure, particularly in developing economies, and growing clinical awareness of advanced treatment options have also supported market expansion.
North America leads the Metaraminol Bitartrate Market due to its robust healthcare infrastructure and strong presence of pharmaceutical manufacturers. Europe follows closely, supported by established hospital networks and standardized clinical guidelines for vasopressor use. The Asia-Pacific region is emerging rapidly, driven by expanding healthcare facilities, increasing patient admissions in critical care units, and rising awareness of emergency cardiovascular treatments across countries such as India and China.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Metaraminol Bitartrate Market is projected to grow from USD 322.5 million in 2024 to USD 437.98 million by 2032, registering a CAGR of 3.9%.
- Rising incidence of hypotension and cardiovascular diseases is fueling consistent demand in hospitals and surgical units.
- Increased adoption of vasopressors in anesthesia and critical care is strengthening market growth.
- Limited availability of high-purity formulations and regulatory hurdles continue to challenge smaller manufacturers.
- North America leads the market, supported by advanced healthcare infrastructure and strong pharmaceutical production.
- Europe maintains steady demand driven by well-structured hospital systems and standardized drug protocols.
- Asia-Pacific is emerging as the fastest-growing region due to rapid healthcare expansion and growing cardiovascular patient base.
Market Drivers
Rising Incidence of Hypotension and Cardiovascular Disorders Increasing Demand for Vasopressors
The growing number of patients suffering from hypotension and cardiovascular conditions has accelerated the demand for effective vasopressor drugs. The Metaraminol Bitartrate Market benefits from its widespread use in stabilizing blood pressure during surgical and critical care situations. It is commonly preferred by anesthesiologists for managing intraoperative hypotension due to its quick onset and predictable response. Hospitals and intensive care units continue to rely on it to ensure patient hemodynamic stability. Increasing healthcare access in developing economies supports market growth. The aging population and rise in cardiac-related complications have increased vasopressor utilization. Manufacturers are expanding production capacity to meet rising hospital and clinical demand. This trend reinforces the need for dependable, fast-acting cardiovascular medications globally.
- For instance, Pfizer invested $750 million in a major expansion of its sterile injectable manufacturing facility in Kalamazoo, Michigan, slated to begin production in 2024, to enhance the global supply of critical injectable medicines. This facility will support increased demand for vasopressors and other essential hospital drugs, incorporating modular aseptic manufacturing technology to ensure high reliability and scalable output.
Increasing Use in Emergency Medicine and Perioperative Care Environments
The rise in critical care admissions has raised the use of metaraminol bitartrate in emergency departments and perioperative settings. It is administered to maintain blood pressure during surgeries and post-anesthesia care. The Metaraminol Bitartrate Market growth is supported by medical institutions prioritizing faster patient stabilization. Anesthesiologists prefer it due to its controlled vasoconstrictive properties and lower risk of cardiac complications compared to alternatives. Growing awareness among healthcare professionals about its clinical effectiveness strengthens adoption. Hospitals are integrating standardized metaraminol protocols in surgical units to reduce intraoperative complications. Enhanced healthcare infrastructure in emerging markets improves access to such essential drugs. This expansion underscores the compound’s value in modern anesthetic practice.
Advancements in Pharmaceutical Formulation and Drug Delivery Technologies
Innovations in drug formulation and delivery systems have improved the efficacy and safety of metaraminol bitartrate. Pharmaceutical firms are investing in developing stable and ready-to-use formulations to enhance shelf life and usability. The Metaraminol Bitartrate Market witnesses growth from increased hospital procurement of prefilled syringes and vials. It reduces medication preparation errors and saves critical time during emergencies. Regulatory approvals for improved formulations have encouraged large-scale production. Research efforts focus on reducing adverse reactions through controlled-release technologies. Pharmaceutical companies are improving packaging standards to maintain sterility and drug potency. These advancements enhance clinical outcomes and ensure consistent therapeutic response across applications.
- For instance, B. Braun launched FDA-approved ready-to-administer injectable products in 2025, including Cefazolin Premix (3 g) in DUPLEX containers announced in March and Tromethamine Injection in EXCEL IV containers announced in September. Both utilize B. Braun’s proprietary container systems to enhance sterility, minimize compounding risks, and improve administration safety in hospital environments.
Expansion of Healthcare Infrastructure and Rising Access to Critical Care Facilities
Rapid healthcare modernization, especially in developing regions, has created new opportunities for vasopressor demand. Governments are investing in ICU expansion and anesthesia equipment upgrades. The Metaraminol Bitartrate Market benefits from improved patient monitoring capabilities that encourage timely drug use. It is increasingly integrated into emergency care guidelines in tertiary hospitals. Better diagnostic accuracy and increased hospital admissions for cardiovascular emergencies drive usage. Training programs for emergency physicians improve drug handling and dosing precision. Growing private hospital networks in Asia-Pacific contribute to higher consumption levels. The trend highlights a global shift toward advanced care and reliable pharmacological management of hypotension.
Market Trends
Growing Shift Toward Ready-to-Use Injectable Formulations for Enhanced Clinical Efficiency
The pharmaceutical sector is focusing on ready-to-use injectable forms that simplify administration and ensure sterility. The Metaraminol Bitartrate Market is witnessing strong interest in prefilled syringes and sealed vials. It reduces dosage errors and shortens preparation time during emergencies. Hospital pharmacies increasingly prefer single-dose packaging for efficiency and safety. Regulatory bodies promote pre-mixed formulations that meet quality standards. Suppliers are optimizing logistics to reduce storage complexity in ICUs. Drug manufacturers are aligning production lines to support ready-to-administer products. This shift strengthens patient safety and supports the global adoption of standardized vasopressor therapies.
- For instance, Baxter International launched FDA-approved ready-to-use norepinephrine bitartrate injections in 2024, including a 16 mg/250 mL strength. The product is designed for treating acute hypotension and supplied in Baxter’s sterile, collapsible Galaxy flexible containers to enhance hospital efficiency.
Rising Integration of Vasopressor Use in Clinical Protocols and Treatment Guidelines
Hospitals are formalizing metaraminol bitartrate usage in treatment protocols for consistent therapeutic outcomes. The Metaraminol Bitartrate Market benefits from inclusion in clinical guidelines for intraoperative and postoperative hypotension. It enables clinicians to follow evidence-based practices in patient care. Standardization across hospitals enhances confidence among practitioners. Training programs for anesthetists focus on precise dosing and risk mitigation. Increased publication of efficacy data in peer-reviewed journals validates clinical performance. Healthcare agencies emphasize uniform administration standards for cardiovascular stability. These institutional practices ensure safer and more predictable use in critical care settings.
Growing Focus on Hospital Automation and Drug Administration Accuracy
Automation in hospital systems is improving drug management precision and patient monitoring. The Metaraminol Bitartrate Market gains traction with smart infusion pumps and digital dose tracking systems. It ensures accurate drug delivery in real time during emergencies. Automated dispensing cabinets reduce human error and improve accountability. Hospitals are adopting software solutions that integrate drug databases for metaraminol. Enhanced interoperability between systems aids in maintaining safety standards. Healthcare technology providers are collaborating with pharmaceutical companies to design compatible drug protocols. This integration promotes efficiency and reinforces quality control in patient treatment environments.
- For instance, B. Braun’s Space® Infusion Pump platform, integrated with electronic medical records in U.S. hospitals, achieved a 98% drug library compliance rate and a 92% reduction in alert overrides, ensuring safer and more precise medication delivery.
Increasing Research Investments in Novel Vasopressor Mechanisms and Combination Therapies
Pharmaceutical research continues to explore metaraminol’s synergistic effects with other cardiovascular drugs. The Metaraminol Bitartrate Market benefits from growing funding for studies on safer and faster-acting vasopressors. It drives innovation in receptor-targeted mechanisms for precise blood pressure regulation. Clinical trials are assessing optimized dose-response relationships for different patient groups. Research organizations are partnering with hospitals to validate efficacy in diverse demographics. Novel formulations aim to improve onset duration and minimize adverse events. Enhanced collaboration between academia and industry accelerates translational drug development. This growing research focus underlines the sector’s commitment to next-generation therapeutic improvements.
Market Challenges Analysis
Supply Chain Disruptions and Regulatory Barriers Affecting Product Availability and Distribution
Pharmaceutical supply chain complexity often limits timely drug delivery across regions. The Metaraminol Bitartrate Market faces hurdles from stringent approval processes and limited manufacturing capacity. It depends heavily on consistent raw material supply and strict regulatory compliance. Disruptions in international logistics create shortages in hospital inventories. Manufacturers face cost pressures from maintaining quality under changing global standards. Regulatory delays in emerging economies slow down product registration timelines. Smaller pharmaceutical firms struggle to meet evolving safety documentation requirements. The combination of regulatory burden and unstable supply affects availability in critical care environments.
Potential Adverse Reactions and Limited Clinical Alternatives Restricting Wider Adoption
Concerns related to side effects and complex dosing requirements hinder broader usage. The Metaraminol Bitartrate Market encounters hesitation from clinicians due to risks of hypertension or reflex bradycardia. It demands close patient monitoring during administration to prevent complications. Limited awareness about dosage titration among less experienced professionals creates usage variability. Competing vasopressors with simpler handling attract attention from healthcare providers. Inadequate training resources in some hospitals restrict optimal use. Ongoing pharmacovigilance studies aim to refine safety profiles and dosing ranges. These factors collectively constrain consistent expansion across healthcare institutions worldwide.
Market Opportunities
Growing Investment in Healthcare Modernization and Expanded Access to Critical Care Treatment
Rising government and private sector investments in healthcare expansion strengthen market prospects. The Metaraminol Bitartrate Market gains from ongoing development of emergency and surgical infrastructure. It aligns with national healthcare programs emphasizing patient safety and cardiac care. Increased funding for critical care units enhances drug adoption in tertiary hospitals. Emerging economies are building stronger supply networks for essential medicines. Healthcare digitization supports efficient drug tracking and storage management. Pharmaceutical collaborations are addressing supply gaps through localized production. These combined efforts create sustained demand for high-quality cardiovascular drugs.
R&D Collaborations and Product Innovation Creating New Growth Opportunities
Joint research initiatives between pharmaceutical companies and medical institutions are fostering innovation. The Metaraminol Bitartrate Market benefits from advanced formulation research that ensures consistent efficacy. It encourages the creation of combination therapies and patient-specific treatment models. Regulatory flexibility for new dosage formats attracts greater investment. Expansion in contract manufacturing supports scalable supply and faster distribution. Pharmaceutical innovators are pursuing bioequivalence studies to enter regulated markets. Growing collaboration with clinical research organizations promotes faster clinical validation. This innovation-oriented landscape is expected to sustain long-term competitiveness and market advancement.
Market Segmentation Analysis:
By Product Type
The Metaraminol Bitartrate Market is categorized into API, solution, powder, and others. Solutions, including injectable forms, dominate due to their extensive use in emergency and surgical care. It remains the preferred format in hospitals for rapid blood pressure restoration. The API segment contributes significantly, driven by pharmaceutical manufacturing and drug compounding. Powder formulations serve research and laboratory applications where stability is prioritized. The “others” category includes specialized preparations tailored for clinical studies and low-volume medical uses.
- For instance, Cambrex announced in October 2025 a $120 million investment to expand its API manufacturing facility in Charles City, Iowa, by 40%, increasing total production capacity to nearly one million liters annually for pharmaceutical-grade active ingredients.
By Type
The market is segmented into purity ≥99% and purity <99%. The ≥99% purity segment holds the largest share, reflecting strict pharmaceutical standards and clinical safety requirements. It is the benchmark grade for injectable vasopressor production. Manufacturers maintain tight quality control to ensure compliance with international pharmacopeia standards. The lower purity segment is primarily used in research and early-stage formulation development. Demand in this segment remains stable due to continuous clinical testing activities.
By Application
The market includes hypotension treatment, injection, pharmaceutical manufacturing, research and development, surgery/emergency medical procedures, and others. Hypotension treatment leads due to widespread adoption in managing acute cardiovascular conditions. It is frequently used during anesthesia and critical care interventions. The injection segment supports demand through emergency medicine and ICU procedures. Pharmaceutical manufacturing and R&D segments contribute through clinical innovation and formulation testing. Surgical and emergency use continues to expand with growing hospital admissions for cardiac care.
- For instance, a 2023 Australian ICU study reported that out of 17,432 patients receiving single-agent vasopressor therapy, 1,963 (11.3%) were administered metaraminol for acute hypotension management.
By End User
End users include hospitals, clinics, pharmacies, and research institutes. Hospitals represent the largest share, supported by continuous use in operating rooms and ICUs. It remains essential in anesthetic practice and emergency response units. Clinics utilize it for perioperative monitoring and short-term cardiovascular support. Pharmacies contribute through distribution and compounding for institutional supply. Research institutes drive long-term demand by conducting clinical studies and exploring new formulation advancements.
Segmentation:
By Product Type:
- API (Active Pharmaceutical Ingredient)
- Solution (including injectable forms)
- Powder
- Others
By Type:
By Application:
- Hypotension Treatment
- Injection
- Pharmaceutical Manufacturing
- Research and Development
- Surgery/Emergency Medical Procedures
- Others
By End User:
- Hospitals
- Clinics
- Pharmacies
- Research Institutes
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America dominates the Metaraminol Bitartrate Market with a market share of 38%. Strong healthcare infrastructure, high prevalence of cardiovascular conditions, and advanced critical care facilities drive its leadership. It benefits from robust regulatory support and increased use of vasopressors in hospitals and emergency departments. Continuous R&D initiatives and rapid adoption of ready-to-use injectable drugs strengthen its position. The U.S. remains the primary contributor, supported by leading pharmaceutical manufacturers and strong hospital purchasing networks. Canada contributes with rising clinical trials and increasing awareness of hypertension management.
Europe holds a market share of 29%, supported by established pharmaceutical manufacturing capabilities and a structured healthcare framework. It demonstrates consistent demand for metaraminol bitartrate in perioperative care and anesthesia. Strong government funding for public hospitals and expanding geriatric populations sustain market growth. The region’s emphasis on patient safety encourages the use of standardized vasopressor formulations. Countries such as Germany, the U.K., and France lead production and consumption due to advanced clinical practices. It benefits from stringent drug quality standards, ensuring steady adoption across medical institutions.
Asia-Pacific accounts for 24% of the Metaraminol Bitartrate Market and represents the fastest-growing regional segment. Growth is driven by expanding hospital infrastructure and rising cardiovascular disease rates in developing economies. China, India, and Japan are major contributors with growing demand in emergency care and surgery units. Increasing investments in pharmaceutical production and domestic drug supply chains enhance regional availability. It experiences rising adoption due to medical training improvements and broader access to anesthesia services. The shift toward modernized healthcare systems reinforces long-term market potential. Latin America and the Middle East & Africa collectively hold 9%, with emerging demand supported by healthcare modernization and gradual hospital expansion.
Key Player Analysis:
- GLAND PHARMA LIMITED
- Fresenius Kabi AG
- Merck & Co., Inc.
- Sigma-Aldrich Corporation
- GD Searle LLC
- SPHINX Scientific Laboratory (Tianjin) Co., Ltd.
- Malladi Drugs & Pharmaceuticals Ltd.
- Changzhou Sunlight Pharmaceutical Co., Ltd.
- Spectrum Chemical Manufacturing Corp.
- Sinoway Chemical Company
- Toronto Research Chemicals Inc.
- Biosynth AG
- Laboratori Alchemia S.p.A.
- Wuhan Fortuna Chemical Co., Ltd.
- Pfizer Inc.
Competitive Analysis:
The Metaraminol Bitartrate Market features a mix of global and regional pharmaceutical players competing on product quality, reliability, and regulatory compliance. Leading companies focus on developing sterile, ready-to-use formulations and expanding supply across hospital networks. It remains competitive due to consistent clinical demand and the need for high manufacturing standards. Firms invest in R&D collaborations to refine drug stability and reduce adverse reactions. Strategic partnerships with distributors and healthcare providers improve accessibility in emerging markets. Companies emphasize compliance with Good Manufacturing Practices to strengthen market presence. The growing preference for injectable forms intensifies rivalry among producers targeting advanced hospital procurement channels.
Recent Developments:
- In January 2025, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. announced that its subsidiary Jinzhou Avanc Pharmaceutical Co., Ltd. received marketing registration approval from China’s National Medical Products Administration (NMPA) for a metaraminol bitartrate injection (1 mL:10 mg). The drug is intended for prevention and treatment of acute hypotensive states during spinal-anesthesia, hemorrhage-related hypotension, surgical complications, cardiogenic shock and septicemia-induced hypotension.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage:
The research report offers an in-depth analysis based on Product Type, Type, Application and End User. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Growing demand for metaraminol bitartrate in anesthesia and critical care will continue to support global adoption.
- Hospitals will remain the leading consumers, driven by expanded ICU capacity and improved emergency care infrastructure.
- Pharmaceutical manufacturers are expected to invest in advanced formulations, focusing on ready-to-use injectable solutions.
- Research initiatives will strengthen understanding of metaraminol’s pharmacological profile, promoting safer administration protocols.
- Strategic partnerships between drug producers and healthcare institutions will enhance market penetration across developing economies.
- Increasing prevalence of cardiovascular disorders will sustain consistent clinical need in both surgical and emergency applications.
- Regulatory support for quality manufacturing standards will encourage broader distribution in regulated markets.
- Technological innovation in drug packaging and delivery systems will improve dosage accuracy and reduce wastage.
- Growing healthcare spending in Asia-Pacific will create new revenue opportunities for local manufacturers.
- Continuous training of healthcare professionals will drive standardized clinical use and ensure better patient outcomes.




