Market Overview:
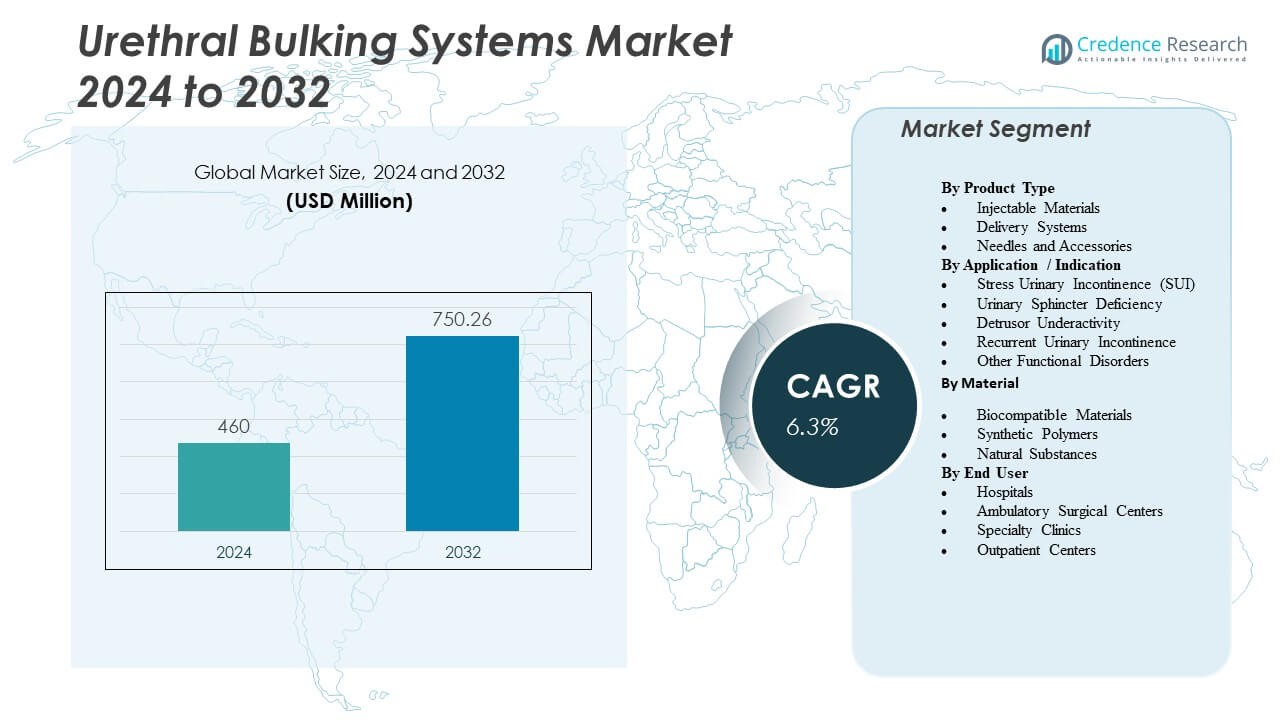
The Urethral Bulking Systems Market is projected to grow from USD 460 million in 2024 to an estimated USD 750.26 million by 2032, with a compound annual growth rate (CAGR) of 6.3% from 2024 to 2032.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Urethral Bulking Systems Market Size 2024 |
USD 460 Million |
| Urethral Bulking Systems Market, CAGR |
6.3% |
| Urethral Bulking Systems Market Size 2032 |
USD 750.26 Million |
Growing awareness of women’s health and advancements in biomaterial technology are major factors fueling the Urethral Bulking Systems Market. Healthcare providers increasingly recommend bulking agents for stress urinary incontinence due to shorter recovery times and reduced surgical risks. Manufacturers are developing biocompatible, long-lasting agents to improve efficacy and patient outcomes. The aging population and growing preference for outpatient treatments are further driving adoption. Expanding reimbursement support and technological innovation are also enhancing procedural accessibility across healthcare systems.
North America leads the market, supported by strong healthcare infrastructure, high diagnosis rates, and rapid adoption of innovative non-surgical treatments. Europe follows with strong clinical research and favorable regulatory environments promoting advanced product use. The Asia-Pacific region is emerging due to improving healthcare facilities, increasing awareness, and expanding investments in women’s health. Countries like Japan, China, and India show strong growth potential. Latin America and the Middle East & Africa are gradually gaining traction through medical infrastructure development and growing patient education.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Urethral Bulking Systems Market is projected to grow from USD 460 million in 2024 to USD 750.26 million by 2032, supported by a 6.3% CAGR.
- Rising urinary incontinence cases among aging women and demand for minimally invasive procedures strengthen treatment adoption across hospitals and outpatient centers.
- Technological advancements in biocompatible bulking agents and improved delivery systems increase long-term treatment success and patient comfort.
- Limited long-term durability of some agents and the need for repeat procedures continue to restrain faster penetration in certain patient groups.
- North America leads due to advanced healthcare infrastructure, high diagnosis rates, and strong acceptance of non-surgical treatments.
- Europe maintains steady growth with strong research activity and favorable regulatory support for new bulking technologies.
- Asia-Pacific shows fast expansion as healthcare access improves and awareness of women’s health conditions increases in emerging economies.
Market Drivers
Rising Prevalence of Urinary Incontinence Among Aging Population
The growing elderly population drives demand for urethral bulking systems due to the rising prevalence of stress and mixed urinary incontinence. Women over 50 years are particularly affected by weakened pelvic muscles and hormonal decline, leading to increased treatment adoption. The Urethral Bulking Systems Market benefits from awareness campaigns that promote early diagnosis and non-surgical interventions. Hospitals report a consistent rise in outpatient consultations for urinary incontinence management. Patients prefer minimally invasive options to avoid the recovery time of surgical procedures. Healthcare professionals recommend urethral bulking as a primary line of defense against mild to moderate leakage. The aging trend across North America and Europe further supports this demand. It continues to drive innovation in material composition and delivery precision.
- For instance, a long-term multicenter study on the Bulkamid® urethral bulking agent (Coloplast/Boston Scientific) followed 388 of 1,200 treated women over seven years, showing 67.1% felt cured or improved after primary treatment, with a 3.5% rate of transient urinary tract infection.
Increasing Adoption of Minimally Invasive and Office-Based Treatments
The market growth is supported by the preference for procedures performed in outpatient or office settings. These techniques reduce hospital stay, minimize complications, and lower overall costs. Patients seek effective treatments with reduced discomfort and downtime. The Urethral Bulking Systems Market is witnessing high acceptance among women avoiding invasive surgery. Physicians prefer advanced delivery devices that enhance control and precision during injections. Healthcare systems in developed nations are expanding training programs to promote these procedures. Newer agents such as polyacrylamide and calcium hydroxyapatite are gaining acceptance for their biocompatibility. It creates a favorable environment for companies offering patient-friendly, low-risk products.
Advancements in Biocompatible Materials and Injection Technology
Innovation in biomaterials significantly enhances product safety and effectiveness. Modern bulking agents deliver improved tissue integration and long-term urethral support. The Urethral Bulking Systems Market benefits from FDA approvals for novel agents ensuring durability and minimal allergic reactions. Research focuses on hydrogel-based compositions that maintain elasticity and stability for longer durations. Manufacturers are developing dual-syringe systems and ergonomic injectors to improve procedural efficiency. Clinical evaluations demonstrate improved patient satisfaction and repeat-procedure success rates. Surgeons increasingly rely on image-guided systems to optimize injection placement. It strengthens confidence among both clinicians and patients in adopting new-generation devices.
- For instance, a seven-year follow-up study on the Bulkamid® polyacrylamide hydrogel reported a mean reduction of 8.6 points in the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF) score, demonstrating sustained symptom improvement and long-term efficacy in women with stress urinary incontinence. The Contigen® collagen implant (Bard/Allergan) also holds FDA approval for treating urinary incontinence caused by intrinsic sphincter deficiency.
Growing Awareness and Expanding Reimbursement Support
Government and private initiatives promoting women’s health play a key role in expanding market adoption. Awareness campaigns highlight the importance of treating urinary incontinence early. The Urethral Bulking Systems Market benefits from favorable reimbursement policies in the U.S. and European countries. Health insurers now include non-surgical interventions for stress urinary incontinence within coverage plans. This financial accessibility encourages patients to undergo early intervention. Clinics also report higher treatment volumes due to reduced procedural costs. Medical associations support the inclusion of urethral bulking systems in standard treatment protocols. It reinforces patient trust and accelerates clinical adoption across healthcare networks.
Market Trends
Integration of Image-Guided and AI-Assisted Delivery Systems
Technological innovation is reshaping the precision and safety of urethral bulking procedures. Image-guided systems enhance visualization and improve placement accuracy during injections. AI-based platforms analyze tissue response to determine ideal bulking volume in real time. The Urethral Bulking Systems Market incorporates these technologies to reduce operator variability and improve outcomes. Hospitals adopt AI-enabled injectors that assist in optimal needle alignment and volume distribution. These tools reduce learning curves for physicians new to the procedure. Integration of smart technology enables data capture for post-procedure tracking. It elevates clinical confidence and boosts efficiency in treatment delivery.
- For instance, Boston Scientific’s Coaptite Injectable Implant uses image-guided delivery and demonstrated, in a multi-center clinical trial, that 63% of patients experienced improvement at 12 months post-procedure. The clinical data also revealed the Coaptite system required significantly less implant volume (average 4 ml) versus the bovine collagen comparator (average 6.8 ml), with improved post-procedural success.
Shift Toward Next-Generation Synthetic and Bioabsorbable Agents
Manufacturers are developing advanced materials that provide superior performance and longevity. Bioabsorbable agents promote tissue regeneration while minimizing risks of migration or rejection. The Urethral Bulking Systems Market is evolving toward sustainable materials that mimic natural tissue properties. Clinical results indicate improved patient comfort and long-term continence restoration. Companies focus on achieving consistent particle size distribution to ensure even dispersion. Newer compositions prevent clumping and allow repeat procedures when necessary. Collaborations between material scientists and clinicians accelerate design improvements. It strengthens the market’s move toward high-performance, durable bulking solutions.
Growing Use of Digital Platforms for Patient Education and Consultation
Digital health tools are improving awareness and accessibility to incontinence treatments. Telemedicine and mobile apps enable virtual consultations and follow-up care. The Urethral Bulking Systems Market benefits from educational campaigns using social media and health portals. Patients can now compare treatments and locate specialists with ease. Hospitals use digital outreach to inform women about non-surgical therapy options. Online forums support patient communities and improve treatment compliance. Healthcare professionals conduct webinars to train on advanced bulking procedures. It fosters better understanding and expands the overall treatment base.
- For instance, Renovia’s Leva Digital Therapeutic System has been used in several U.S. clinics and empowers women with stress urinary incontinence to perform biofeedback-enhanced pelvic floor training at home using a Bluetooth-enabled vaginal sensor paired with a smartphone app.
Rise in Outpatient Clinics and Ambulatory Surgical Centers
The healthcare shift toward outpatient care fuels adoption of in-office treatments. Ambulatory surgical centers provide cost-effective environments with shorter waiting times. The Urethral Bulking Systems Market is gaining traction in these settings due to simplified logistics and rapid patient turnover. Hospitals collaborate with outpatient networks to expand access to female urology services. This approach helps manage the growing number of incontinence cases efficiently. Physicians find outpatient environments ideal for low-risk, non-anesthetic procedures. Equipment standardization and portable injection systems further enhance accessibility. It encourages procedural expansion across urban and semi-urban regions.
Market Challenges Analysis
Limited Long-Term Efficacy and Need for Repeat Procedures
Despite technological improvements, achieving long-lasting continence remains difficult. Many patients require repeat injections within a few years to maintain effectiveness. The Urethral Bulking Systems Market faces challenges related to variability in clinical outcomes. Some agents lose bulking volume over time due to resorption or tissue migration. Inconsistent injection depth can also lead to uneven results. Physicians must balance between long-term safety and durability when selecting agents. Patients often experience reduced satisfaction due to repeat visits. It drives ongoing research into materials that retain volume stability for extended periods.
Stringent Regulatory Approvals and Clinical Evidence Requirements
Regulatory processes for novel bulking agents demand extensive safety and efficacy data. Gaining approval for innovative formulations is both time-consuming and costly. The Urethral Bulking Systems Market experiences delays in product commercialization due to complex clinical validation. Regional differences in device classification and trial design further hinder global launches. Smaller firms face difficulties meeting stringent documentation and post-market surveillance obligations. Physicians hesitate to adopt new materials until long-term results are published. This environment limits rapid technological diffusion across healthcare systems. It slows the pace of innovation despite growing clinical demand.
Market Opportunities
Expanding Focus on Women’s Health and Preventive Urology Programs
Governments and non-profit organizations are prioritizing women’s health as part of preventive care strategies. Campaigns encourage early diagnosis of urinary incontinence and support access to non-invasive treatments. The Urethral Bulking Systems Market can benefit from integration into community health programs. Hospitals are establishing specialized urogynecology units to handle rising patient volumes. Training initiatives aim to enhance procedural competence among clinicians in emerging countries. Educational outreach strengthens awareness about treatment options among women hesitant to seek help. Health technology investments provide infrastructure for diagnosis and therapy. It creates opportunities for sustained market penetration across developing economies.
Product Innovation and Collaboration with Research Institutions
Partnerships between medical device companies and research institutes are fostering innovation. Joint projects focus on developing biodegradable materials and customized delivery systems. The Urethral Bulking Systems Market gains advantage from cross-industry collaboration in biomaterial science. Universities contribute through preclinical testing and performance analysis of new compounds. Manufacturers leverage this data to accelerate regulatory submissions. Clinical trials supported by public-private initiatives improve confidence among healthcare professionals. Technology transfer programs enable start-ups to scale novel solutions efficiently. It supports a competitive ecosystem where innovation translates into real clinical outcomes.
Market Segmentation Analysis:
By Product Type
Injectable materials hold the largest share of the Urethral Bulking Systems Market due to their critical role in restoring urethral closure and function. Collagen-based agents, synthetic polymers, and hydrogel formulations lead usage for stress urinary incontinence treatment. Delivery systems are evolving with ergonomic injectors and image-guided precision to enhance procedural outcomes. Needles and accessories remain essential for controlled administration and depth accuracy during procedures. Manufacturers focus on developing devices that reduce complications and improve patient comfort. It continues to drive product innovation and clinical efficiency across healthcare settings.
By Application / Indication
Stress urinary incontinence (SUI) represents the dominant indication due to its high prevalence among women. The Urethral Bulking Systems Market benefits from growing clinical preference for minimally invasive management of SUI. Urinary sphincter deficiency and detrusor underactivity segments are expanding with advances in material longevity and repeatability. Recurrent urinary incontinence cases increasingly use bulking systems to improve urethral resistance after surgical failure. Other functional disorders are gradually gaining attention as new clinical studies validate efficacy across diverse patient populations. It supports a broader adoption base within urology and urogynecology practices.
- For instance, the FDA Summary of Safety and Effectiveness Data for Bulkamid (P170023) reports that 228 women were enrolled in the pivotal U.S. clinical study, and 87.7% of participants completed the 12-month follow-up. The study showed a mean reduction of 2.6 urinary incontinence episodes per day based on patient voiding diaries.
By Material
Biocompatible materials lead this segment, offering longer durability and reduced tissue reaction. The Urethral Bulking Systems Market emphasizes hydrogel-based and calcium hydroxyapatite compounds for sustained volume maintenance. Synthetic polymers show improved mechanical stability and low migration risk. Natural substances appeal to patients seeking biologically safe compositions with proven clinical tolerance. Research continues to refine composite materials balancing elasticity and integration. It reinforces trust among physicians focused on safety and treatment consistency.
By End User
Hospitals remain the leading end users, supported by access to advanced imaging and multidisciplinary teams. The Urethral Bulking Systems Market expands rapidly within ambulatory surgical centers due to reduced costs and faster recovery times. Specialty clinics deliver personalized treatment and follow-up care, improving patient outcomes. Outpatient centers are emerging as preferred venues for repeat procedures, supported by portable injection systems and streamlined workflows. It strengthens accessibility for women seeking discreet, efficient, and affordable treatment options.
- For instance, the U.K. National Health Service (NHS) recognizes urethral bulking as a standard day-case procedure for stress urinary incontinence in women, typically performed under local anesthesia with rapid recovery and minimal postoperative complications, according to NICE guidance and NHS treatment protocols.
Segmentation:
By Product Type
- Injectable Materials
- Delivery Systems
- Needles and Accessories
By Application / Indication
- Stress Urinary Incontinence (SUI)
- Urinary Sphincter Deficiency
- Detrusor Underactivity
- Recurrent Urinary Incontinence
- Other Functional Disorders
By Material
- Biocompatible Materials
- Synthetic Polymers
- Natural Substances
By End User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Outpatient Centers
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America dominates the Urethral Bulking Systems Market with a market share of 41%. The region benefits from advanced healthcare infrastructure, high diagnostic awareness, and favorable reimbursement structures. The United States leads due to the presence of key manufacturers and strong clinical adoption of minimally invasive treatments. Ongoing research on biomaterial innovation further strengthens its leadership position. Canada supports growth through government initiatives promoting women’s urological health. It continues to attract investments in outpatient centers and specialized clinics focused on incontinence management.
Europe holds a 29% share, driven by well-established healthcare systems and clinical research advancements. Countries such as Germany, France, and the United Kingdom are major contributors to market expansion. Regulatory support and early adoption of bio-compatible bulking agents have boosted procedure rates. Growing awareness of female urinary disorders encourages more patients to opt for non-surgical interventions. The European market benefits from continuous technology development and strategic partnerships. It remains a vital hub for innovation and device testing before global rollouts.
Asia-Pacific accounts for 21% of the market and is the fastest-growing regional segment. Rising awareness of urinary incontinence and improvements in healthcare access are driving strong regional adoption. Japan, China, and India are emerging markets with increasing patient volumes and healthcare investments. Expanding women’s health programs and urban lifestyle changes support higher treatment rates. The region witnesses growing entry of international players establishing local distribution networks. It is expected to gain more share over the next decade with ongoing infrastructure upgrades. Latin America and the Middle East & Africa collectively represent 9% and continue to develop through gradual healthcare modernization.
Key Player Analysis:
- Boston Scientific Corporation
- Coloplast Corp.
- CR Bard
- Laborie Medical Technologies
- Merz Aesthetics
- Axonics (Bulkamid product)
- Uroplasty, Inc. (now part of Laborie)
- Stryker Corporation
- Cook Medical
- Braun Melsungen AG
- Neomedic International
- Medtronic plc
- Prometheus Group
- ASCENTX Medical, Inc.
- Anepro Medical
Competitive Analysis:
The Urethral Bulking Systems Market features a moderately consolidated landscape with key players focusing on technology-driven differentiation and product safety. Leading companies include Contura International (Bulkamid), Boston Scientific (Coaptite), Cogentix Medical (Macroplastique), Coloplast, and SRS Medical. These firms emphasize R&D to enhance biocompatibility, durability, and procedural precision. Strategic partnerships with hospitals and clinics support broader adoption of advanced delivery systems. Emerging players are introducing hydrogel-based materials to improve patient outcomes. It maintains strong competition based on clinical evidence, regulatory approvals, and distribution efficiency. Companies aim to strengthen brand trust through consistent performance validation and training initiatives for healthcare professionals.
Recent Developments:
- In November 2024, Boston Scientific finalized the acquisition of Axonics, a move that enhances its offerings in urinary and bowel dysfunction treatments, including the Bulkamid urethral bulking agent. This acquisition supports Boston Scientific’s strategy to provide comprehensive solutions for stress urinary incontinence, with Bulkamid joining a portfolio that targets various stages and severities of incontinence.
- In January 2024, Boston Scientific Corporation announced a definitive agreement to acquire Axonics, Inc., a medical technology company specializing in devices for urinary and bowel dysfunction, including the Bulkamid Urethral Bulking System. This strategic acquisition, valued at approximately $3.7 billion, enhances Boston Scientific’s portfolio within the urology segment and positions the company as a significant player in the urethral bulking systems market.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage:
The research report offers an in-depth analysis based on Product Type, Application / Indication, Material, End User. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Increasing preference for non-surgical procedures will continue to boost market adoption among aging women.
- Advancements in biomaterials will enhance safety, biocompatibility, and long-term treatment durability.
- AI-assisted injection systems and image-guided delivery tools will improve procedural precision.
- Expanding outpatient and ambulatory centers will strengthen accessibility and reduce healthcare costs.
- Strategic collaborations between device manufacturers and research institutes will accelerate product innovation.
- Growing public awareness campaigns on urinary incontinence will increase early diagnosis rates.
- Emerging economies in Asia-Pacific and Latin America will experience rapid adoption through healthcare modernization.
- Regulatory approvals for new bulking agents will expand treatment options and patient confidence.
- Continuous physician training and education programs will enhance clinical expertise and procedural outcomes.
- Rising investments in women’s health and preventive urology will reinforce long-term industry stability.




