Market Overview:
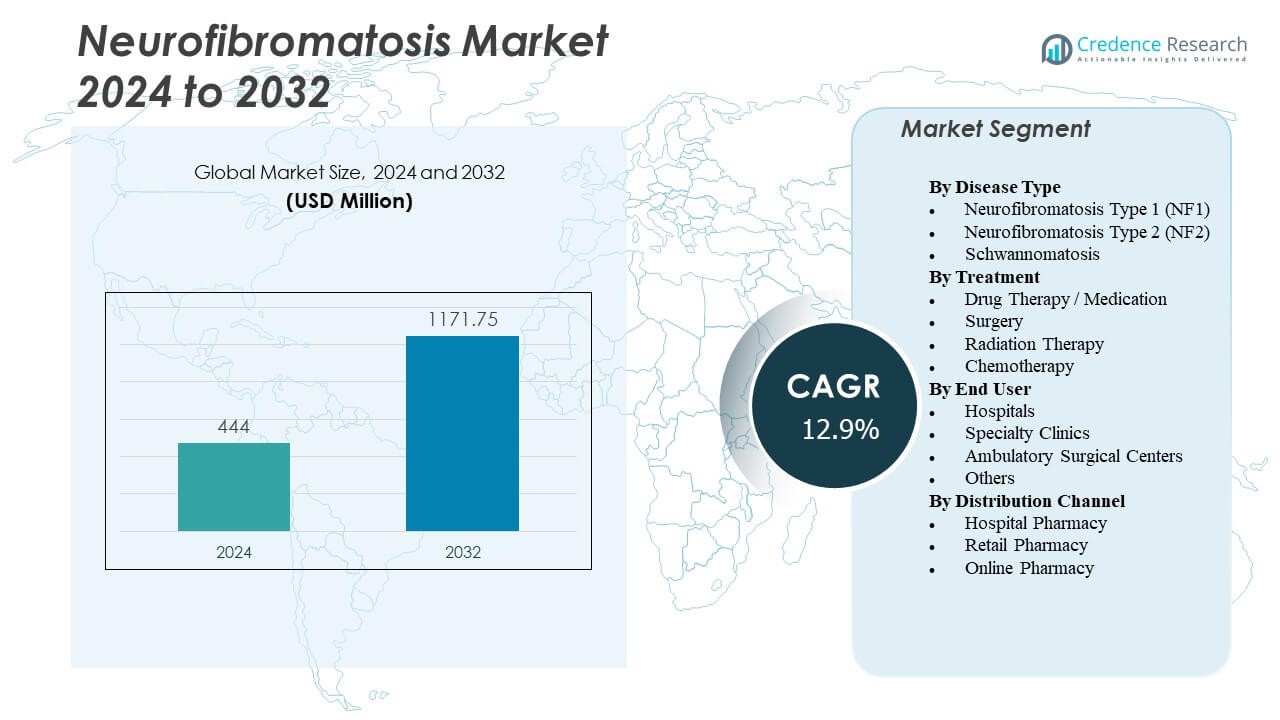
The Neurofibromatosis Market is projected to grow from USD 444 million in 2024 to an estimated USD 1,171.75 million by 2032, with a compound annual growth rate (CAGR) of 12.9% from 2024 to 2032.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Neurofibromatosis Market Size 2024 |
USD 444 Million |
| Neurofibromatosis Market, CAGR |
12.9% |
| Neurofibromatosis Market Size 2032 |
USD 1,171.75 Million |
Rising demand for early diagnosis and effective treatment options fuels growth in the Neurofibromatosis Market. Advances in molecular diagnostics, gene therapy, and targeted drug development allow clinicians to tailor treatments to patient-specific genetic profiles. Supportive regulatory frameworks for orphan drugs encourage innovation and accelerate product approvals. Patient advocacy groups increase awareness, promoting early intervention and therapy adoption. The integration of multidisciplinary care models ensures comprehensive management, while collaborations between healthcare providers and research institutions strengthen clinical capabilities. Investment in research pipelines continues to expand therapeutic options and improve patient outcomes.
North America leads the Neurofibromatosis Market due to advanced healthcare infrastructure, high R&D investment, and early adoption of novel therapies. Europe follows with well-established rare disease policies, specialized clinical centers, and robust clinical trial networks. Asia-Pacific is emerging rapidly as countries like Japan, China, and India expand genetic testing, improve diagnostics, and increase patient access. Latin America and the Middle East & Africa show gradual growth through healthcare infrastructure improvements and rising awareness. Regional focus on early diagnosis and treatment availability drives market expansion and supports long-term adoption globally.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Neurofibromatosis Market is projected to grow from USD 444 million in 2024 to USD 1,171.75 million by 2032, at a CAGR of 12.9%.
- Rising demand for early diagnosis and targeted therapies drives market growth and adoption globally.
- Advances in molecular diagnostics, gene therapy, and personalized treatment enhance patient outcomes.
- Supportive regulatory frameworks for orphan drugs encourage innovation and faster product approvals.
- High treatment costs and limited access in certain regions restrain market expansion.
- North America leads the market, followed by Europe, while Asia-Pacific emerges with growing adoption.
- Collaborations between healthcare providers, research institutions, and patient advocacy groups strengthen clinical capabilities and awareness.
Market Drivers
Advancements in Genetic Research and Diagnostic Technologies
Rapid advancements in molecular genetics and imaging technology have improved early diagnosis and disease characterization in neurofibromatosis. Genetic sequencing tools enable identification of NF1, NF2, and Schwannomatosis mutations with higher accuracy. These tools help clinicians tailor therapies to specific genetic variants. Pharmaceutical companies invest heavily in biomarker discovery to refine patient selection. The Neurofibromatosis Market benefits from precision medicine approaches that enhance treatment outcomes. Hospitals adopt advanced MRI and PET scans for better tumor visualization. Research institutions strengthen collaborations to validate new diagnostic biomarkers. Early diagnosis encourages proactive patient management and supports clinical research enrollment.
- For example, Illumina’s NovaSeq X Series platform, launched in late 2022, can sequence over 20,000 whole genomes per year. It delivers approximately 2.5‑fold higher throughput compared to previous systems, enabling significant advances in rare disease genetic research.
Rising Prevalence and Increased Awareness of Rare Neurological Disorders
The growing number of diagnosed neurofibromatosis cases has increased healthcare attention toward rare disorders. Patient advocacy groups raise awareness through campaigns, improving detection rates and funding support. Governments fund awareness programs that promote genetic counseling and education. Healthcare professionals receive better training to recognize early symptoms. Pharmaceutical pipelines expand due to rising patient populations. The Neurofibromatosis Market gains momentum from community-based initiatives promoting early diagnosis. Global NGOs facilitate cross-border research collaboration to strengthen understanding. Awareness contributes to earlier intervention and improved patient outcomes across clinical settings.
- For instance, the Children’s Tumor Foundation (CTF) organized the “Shine a Light on NF” campaign for World NF Awareness Day in May 2024, demonstrating tangible reach by illuminating nearly 400 world-famous landmarks in blue and green—the official NF colors—across multiple continents to expand global public awareness for neurofibromatosis and related conditions.
Growing Pipeline of Targeted Therapies and Orphan Drug Approvals
Research investment in targeted therapies has transformed treatment approaches for neurofibromatosis. FDA and EMA regulatory incentives encourage orphan drug development. Pharmaceutical firms focus on MEK inhibitors, gene therapies, and molecular signaling modulators. Selumetinib approval for NF1 tumors set a precedent for similar drugs. The Neurofibromatosis Market benefits from accelerated approvals that reduce time-to-market. Collaborative programs between biotech firms and universities generate robust clinical data. Orphan drug policies attract investments from mid-sized biotech innovators. Focus on tumor reduction and quality-of-life improvement drives continued product development.
Supportive Healthcare Infrastructure and Strategic Collaborations
Healthcare systems strengthen rare disease programs with advanced infrastructure and funding support. Governments expand reimbursement policies to include genetic testing and therapies. Research networks connect hospitals, labs, and biotech firms for joint trials. The Neurofibromatosis Market gains traction from patient registries and multicenter studies. Partnerships with advocacy organizations improve access to experimental therapies. Academic centers develop multidisciplinary care models involving neurologists and geneticists. Collaborations improve patient outcomes and speed up therapeutic innovation. Funding incentives support translational research that bridges discovery and commercial development.
Market Trends
Adoption of Gene Therapy and CRISPR-Based Approaches
Gene-editing technologies are redefining therapeutic research in neurofibromatosis. CRISPR and viral vector platforms offer pathways to correct NF1 and NF2 mutations. Research institutions lead clinical programs focused on restoring normal protein function. The Neurofibromatosis Market witnesses rapid adoption of genetic correction therapies in early-stage trials. Companies invest in scalable gene delivery systems that ensure safety and efficacy. Hospitals begin integrating genetic repair tools into long-term care planning. Patient registries help monitor post-therapy outcomes and efficacy patterns. This technological integration strengthens prospects for personalized medicine.
- For example, Beam Therapeutics announced positive initial results from its Phase 1/2 clinical trial of BEAM‑302 for alpha-1 antitrypsin deficiency in March 2025. The therapy showed increases in functional protein levels and was well-tolerated across dose cohorts.
Integration of Artificial Intelligence in Diagnosis and Imaging Analysis
Artificial intelligence tools enable precise tumor detection and progression tracking in neurofibromatosis patients. Deep learning algorithms interpret MRI and CT data for accurate growth assessment. AI-driven analytics reduce diagnostic errors and optimize treatment plans. The Neurofibromatosis Market benefits from predictive models that forecast disease progression. Healthcare systems deploy AI-based workflow tools for faster radiology reporting. Startups design software that identifies mutation patterns linked to tumor behavior. Hospitals adopt AI-assisted dashboards for multidisciplinary coordination. Automation in diagnosis enhances both speed and reliability of clinical outcomes.
Expansion of Patient-Centric Care and Telemedicine Platforms
Digital transformation improves accessibility and continuity of care for neurofibromatosis patients. Telemedicine platforms connect patients with specialists in real time. The Neurofibromatosis Market experiences growth from virtual consultations and digital monitoring tools. Wearable sensors track nerve pain and motor function remotely. Health apps facilitate symptom reporting and treatment adherence. Clinics expand online genetic counseling services to underserved regions. Integration of telehealth into rare disease management improves patient retention. Remote care technology enhances convenience and long-term patient engagement.
Growing Academic and Industry Collaborations for Clinical Trials
Collaborations between universities, hospitals, and biopharma firms accelerate therapy validation. Shared databases improve trial recruitment and monitoring efficiency. The Neurofibromatosis Market advances through multinational studies testing targeted molecules. Research alliances increase publication output and enhance clinical transparency. Funding agencies prioritize consortium-led programs addressing genetic and molecular pathways. These partnerships ensure consistency in data collection across populations. Companies leverage academic expertise for trial design and endpoint optimization. Cross-sector collaboration remains central to accelerating novel drug approval.
- For instance, the multinational selumetinib clinical trials for neurofibromatosis type 1 led by the Children’s Tumor Foundation, SpringWorks Therapeutics, and Johns Hopkins University are registered across numerous global sites and have published peer-reviewed results demonstrating safety and efficacy in reducing neurofibroma tumor volume in pediatric patients.
Market Challenges Analysis
High Cost of Treatment and Limited Reimbursement Coverage
The cost burden of lifelong neurofibromatosis management remains a major concern for patients. Specialized drugs and diagnostic imaging raise financial barriers. Limited insurance coverage restricts access to advanced therapies in many regions. The Neurofibromatosis Market faces resistance due to pricing constraints in developing economies. Hospitals struggle to fund gene therapy and long-term genetic counseling. Pharmaceutical companies encounter hurdles in reimbursement negotiations. Rare disease designations often involve limited pricing flexibility. Affordability issues slow adoption despite clinical effectiveness and innovation progress.
Low Awareness and Uneven Access to Specialized Care
Unequal access to skilled professionals and treatment centers limits care quality. Many regions lack genetic counseling services or diagnostic laboratories. The Neurofibromatosis Market suffers from fragmented patient identification systems. Rural areas remain underserved, delaying early detection. Public awareness remains low despite advocacy efforts. Healthcare infrastructure gaps in low-income nations hinder timely treatment. Physicians outside major hospitals face limited training in rare disease management. These disparities create inconsistent clinical outcomes and prolong diagnostic timelines.
Market Opportunities
Rising Investments in Research and Biotechnology Innovation
Expanding funding for rare disease research supports breakthroughs in neurofibromatosis management. Governments and private investors back gene editing and molecular therapy startups. The Neurofibromatosis Market benefits from growing venture capital interest in orphan drug research. Strategic mergers and acquisitions enhance access to genetic technology platforms. Biotech firms explore next-generation delivery systems to improve therapeutic precision. International consortia launch collaborative projects that target new molecular pathways. Expanding patent portfolios attract partnerships with global pharmaceutical leaders. Increased funding accelerates translation of laboratory findings into commercial applications.
Expanding Focus on Personalized and Preventive Care Models
Personalized medicine shifts focus toward customized therapies based on individual genetic profiles. Diagnostic advancements allow identification of specific NF subtypes for tailored interventions. The Neurofibromatosis Market gains from predictive models that forecast treatment response. Hospitals design preventive programs to slow disease progression. Healthcare systems integrate genetic counseling into primary care settings. Pharmaceutical companies test combination therapies for complex symptom management. Data analytics improve long-term monitoring and treatment adherence. Broader application of precision medicine unlocks new growth potential in rare neurological disorders.
Market Segmentation Analysis:
By Disease Type
The Neurofibromatosis Market is segmented by disease type into NF1, NF2, and Schwannomatosis. NF1 accounts for the majority of cases due to its higher prevalence and early onset in childhood. NF2 involves vestibular schwannomas and presents complex clinical management challenges. Schwannomatosis remains rare but drives demand for specialized diagnostics and treatment. Each type requires tailored care pathways, influencing therapeutic choices and healthcare resource allocation. It supports the development of targeted drugs and personalized treatment strategies, fueling innovation within the market.
- For instance, AstraZeneca’s selumetinib was approved by the U.S. FDA for pediatric NF1 in 2020 after SPRINT phase II trials showed a confirmed partial response (≥20% tumor volume reduction) in 66% of children with inoperable symptomatic plexiform neurofibromas, as published in The Lancet and recognized by regulatory bodies.
By Treatment
Treatment segmentation includes drug therapy, surgery, radiation therapy, and chemotherapy. Drug therapy remains prominent due to ongoing development of MEK inhibitors and targeted molecular treatments. Surgery addresses tumor removal and symptomatic relief for severe manifestations. Radiation therapy and chemotherapy are applied selectively in aggressive or recurrent cases. The Neurofibromatosis Market leverages these modalities to improve patient outcomes and expand therapeutic options. Integration of multimodal approaches supports better disease management and clinical adoption.
- For instance, SpringWorks Therapeutics’ mirdametinib achieved a 41% confirmed objective response rate (≥20% tumor reduction) in adults with NF1-associated plexiform neurofibromas in its pivotal ReNeu Phase IIb trial, with response rates published in 2024 by the company and corroborated by regulatory submissions and peer-reviewed abstracts.
By End User
Hospitals, specialty clinics, ambulatory surgical centers, and other healthcare facilities form the end-user segmentation. Hospitals dominate due to advanced infrastructure and access to multidisciplinary care teams. Specialty clinics focus on rare disease expertise and patient monitoring. Ambulatory centers provide procedural support and outpatient management. It allows healthcare providers to optimize patient care pathways and improve accessibility across regions.
By Distribution Channel
Distribution is categorized into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies ensure immediate availability for inpatient treatment. Retail pharmacies expand access in urban and semi-urban areas. Online pharmacies provide convenience and broader reach for ongoing therapy adherence. The Neurofibromatosis Market benefits from these channels to enhance treatment accessibility and patient compliance.
Segmentation:
By Disease Type
- Neurofibromatosis Type 1 (NF1)
- Neurofibromatosis Type 2 (NF2)
- Schwannomatosis
By Treatment
- Drug Therapy / Medication
- Surgery
- Radiation Therapy
- Chemotherapy
By End User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
The Neurofibromatosis Market in North America commands the largest share, estimated at approximately 36% of global value. The region offers advanced healthcare infrastructure, robust orphan drug incentives, and high awareness among practitioners and patients. It leads pipeline activity and therapy approvals, supporting continuous market expansion. Reimbursement frameworks and rare‑disease networks bolster market penetration and drive uptake. The presence of key biopharma players further reinforces regional dominance.
Europe holds roughly 28% of the global market share. It benefits from well‑established rare disease policies, centralized regulatory pathways, and strong clinical research centers. Many European countries have national registries for neurofibromatosis and support specialized multidisciplinary care centers. It attracts both global and local pharma investment for novel therapies and diagnostic tools. Patient advocacy groups in the region drive early diagnosis initiatives which enhance treatment uptake and optimize market growth prospects.
Asia‑Pacific accounts for about 20% of the total market share and shows the fastest growth trajectory. Countries such as China, India, Japan and Australia invest in healthcare expansion, genetic testing capabilities and rare disease treatment access. It experiences improved diagnosis rates for neurofibromatosis and rising demand for targeted therapies. Emerging economies within this region adopt telemedicine and patient‑monitoring platforms that support outreach. Middle East & Africa and Latin America together represent combined share of around 16%, with growth driven by healthcare infrastructure upgrades and increasing awareness.
Key Player Analysis:
- AstraZeneca
- Array BioPharma
- SpringWorks Therapeutics
- Takeda Pharmaceutical Company
- Pfizer Inc.
- Novartis AG
- Merck & Co., Inc.
- Sanofi S.A.
- Johnson & Johnson
- Hoffmann-La Roche Ltd.
- NFlection Therapeutics
- Fosun Pharmaceutical
- Healx Limited
- Teva Pharmaceutical Industries Ltd.
Competitive Analysis:
The Neurofibromatosis Market features a competitive landscape with major pharmaceutical and biotechnology firms driving innovation and commercialization. Key players include AstraZeneca plc, Novartis AG, Pfizer Inc., Takeda Pharmaceutical Company Limited and SpringWorks Therapeutics Inc.These companies concentrate on pipeline development, orphan‑drug indications and strategic acquisitions to secure market positioning. It also sees emerging biotech firms challenging incumbents through specialty therapies and gene‑editing approaches. Firms differentiate by therapeutic modality, geographic reach and partnerships with advocacy groups or academic institutions. The competitive pressure drives pricing strategies, trial design innovation and faster go‑to‑market cycles. Strong IP portfolios and regulatory incentives strengthen leadership status of major players. Smaller companies focus on niche segments or combination treatments to capture unmet needs.
Recent Developments:
- In October 2025, AstraZeneca’s rare disease division Alexion received European Commission approval for Koselugo (selumetinib) for adults with neurofibromatosis type 1 exhibiting symptomatic, inoperable plexiform neurofibromas, marking a significant milestone for neurofibromatosis treatment in the EU.
- In July 2025, Merck KGaA concluded the acquisition of SpringWorks Therapeutics for $3.4 billion, gaining access to GOMEKLI (mirdametinib), the FDA-approved therapy for NF1 plexiform neurofibromas, along with other innovative rare tumor therapeutics, bolstering Merck’s rare disease strategy.
- In February 2025, SpringWorks Therapeutics announced FDA approval of GOMEKLI™ (mirdametinib) for the treatment of adult and pediatric patients with NF1-related plexiform neurofibromas, introducing a new option for families facing this disease.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage:
The research report offers an in-depth analysis based on Disease Type, Treatment, End User and Distribution Channel. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- The Neurofibromatosis Market will expand due to rising adoption of targeted therapies and gene‑based treatments.
- Expansion of genetic testing and diagnostic capabilities will accelerate early disease detection and intervention.
- Investment in orphan drug development will create new therapeutic options and improve patient outcomes.
- Regional growth will be supported by increasing awareness programs and patient advocacy initiatives.
- Telemedicine and digital monitoring solutions will enhance accessibility for patients in remote areas.
- Biopharmaceutical collaborations and partnerships will streamline research, clinical trials, and commercialization.
- Personalized medicine approaches will drive demand for customized treatment plans tailored to genetic profiles.
- Emerging markets will gain traction due to improving healthcare infrastructure and adoption of advanced therapies.
- Clinical trial expansion across multiple geographies will support rapid therapy approval and market penetration.
- Continuous innovation in multidisciplinary care and supportive therapies will strengthen overall market adoption.




