Market Overview
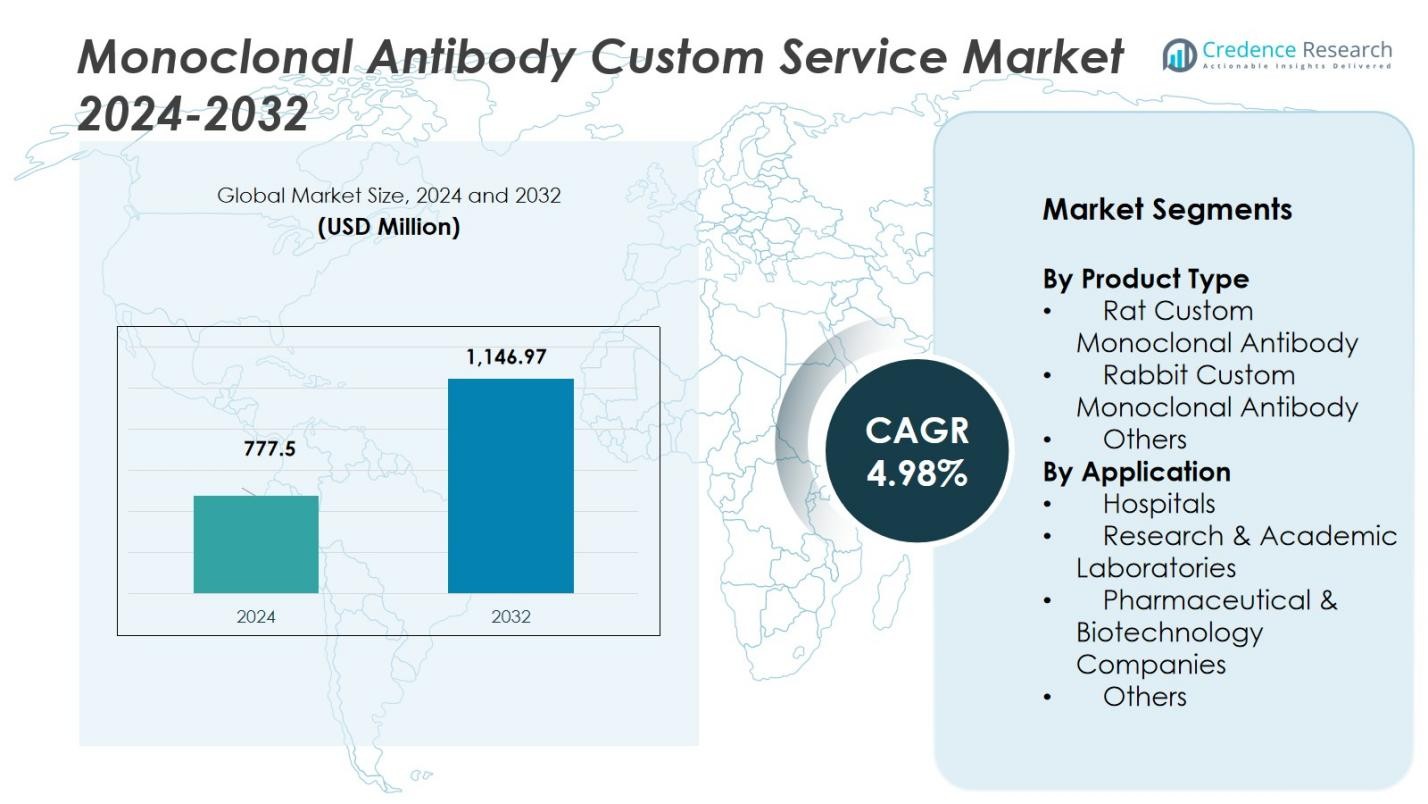
The Monoclonal Antibody Custom Service Market was valued at USD 777.5 million in 2024 and is projected to reach USD 1,146.97 million by 2032, growing at a CAGR of 4.98% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Monoclonal Antibody Custom Service Market Size 2024 |
USD 777.5 Million |
| Monoclonal Antibody Custom Service Market, CAGR |
4.98% |
| Monoclonal Antibody Custom Service Market Size 2032 |
USD 1,146.97 Million |
The Monoclonal Antibody Custom Service Market is dominated by major players including Thermo Fisher Scientific Inc., GenScript, Abcam Plc, Bio‑Rad Laboratories Inc., and Kaneka Eurogentec S.A. These companies offer a broad range of services, utilizing advanced antibody engineering technologies to support drug development and research in the pharmaceutical and biotechnology sectors. North America holds the largest market share, contributing over 44%, driven by a strong pharmaceutical ecosystem, substantial government funding for biotech R&D, and high demand for personalized therapies. Europe follows with a notable share, bolstered by well-established research institutions and regulatory frameworks that support monoclonal antibody innovation. The Asia Pacific region is rapidly emerging as a key growth area, fueled by expanding healthcare infrastructure, rising demand for biotech products, and the increasing trend of outsourcing to cost-effective markets.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Monoclonal Antibody Custom Service Market was valued at USD 777.5 million in 2024 and is projected to reach USD 1,146.97 million by 2032, growing at a CAGR of 4.98% during the forecast period.
- The market is primarily driven by the increasing demand for personalized medicine, advancements in antibody engineering, and the expansion of biopharmaceutical applications.
- Key trends include the rise of antibody-drug conjugates (ADCs) and growing collaborations between research institutions and biopharma companies, further fueling market growth.
- The market faces restraints such as the high cost of custom monoclonal antibody production and regulatory compliance challenges, which may limit growth, especially for smaller players.
- Regionally, North America leads the market with over 44% market share, followed by Europe, while Asia Pacific is emerging as a key growth region with significant potential due to rising healthcare investments and biotech manufacturing expansions.
Market Segmentation Analysis:
By Product Type:
The Monoclonal Antibody Custom Service Market is segmented by product type, including Rat Custom Monoclonal Antibodies, Rabbit Custom Monoclonal Antibodies, and Others. Among these, Rabbit Custom Monoclonal Antibodies dominate the market, holding a significant market share of approximately 55%. This dominance is driven by the superior specificity and affinity of rabbit antibodies, making them ideal for applications in diagnostics and therapeutic development. The growing demand for high-quality antibodies for research and drug discovery further propels the market for rabbit-derived antibodies, driving their widespread adoption across industries.
- For example, GenScript launched its “MonoRab™” custom rabbit monoclonal antibody service explicitly on the basis that rabbit antibodies “are well known for their high affinity, specificity, diversity, and their ability to recognise hidden, hard‑to‑target epitopes”.
By Application:
The Monoclonal Antibody Custom Service Market is also segmented by application, with key sectors including Hospitals, Research & Academic Laboratories, Pharmaceutical & Biotechnology Companies, and Others. The Pharmaceutical & Biotechnology Companies segment leads the market with a share of around 40%. This growth is fueled by the increasing need for monoclonal antibodies in drug development, personalized medicine, and immunotherapy. The demand for tailored antibody solutions in these sectors, combined with the expanding focus on biopharmaceutical innovation, continues to drive the growth and dominance of this segment in the market.
- For instance, the Merck Group is heavily involved in the science of personalized cancer therapies, leveraging internal research and development, as well as collaborations, to engineer and develop monoclonal antibodies (mAbs) and related modalities like antibody-drug conjugates (ADCs) that target specific antigens.
Key Growth Drivers
Increasing Demand for Personalized Medicine
The growing emphasis on personalized medicine is a major driver of the Monoclonal Antibody Custom Service Market. As the healthcare industry shifts towards individualized treatments, there is a rising need for highly specific antibodies that can target particular diseases or genetic profiles. Custom monoclonal antibodies are essential for these tailored therapies, as they can be designed to fit specific patient needs, driving significant demand across pharmaceutical and biotechnology sectors. This trend is expected to continue as more therapies focus on precision medicine, propelling market growth.
- For instance, Roche has advanced the use of bispecific monoclonal antibodies tailored for specific cancer antigens, enhancing treatment precision in oncology.
Advancements in Biotechnology and Research
Ongoing advancements in biotechnology, coupled with an expanding research landscape, are significantly contributing to the growth of the Monoclonal Antibody Custom Service Market. Innovations in antibody engineering, such as improvements in antibody affinity and stability, are enabling the development of more effective therapies. Additionally, the growing number of academic and research institutions focused on antibody-based diagnostics and therapeutics is further increasing the demand for customized antibodies. As research continues to uncover new therapeutic targets, the market for monoclonal antibody services is projected to expand.
- For instance, Genentech’s advancements in monoclonal antibody development have led to the creation of Rituxan, a monoclonal antibody used to treat lymphoma and rheumatoid arthritis, which has successfully treated over 2 million patients worldwide.
Expansion of Biopharmaceutical Applications
The rapid expansion of biopharmaceutical applications is another key driver for the Monoclonal Antibody Custom Service Market. Monoclonal antibodies are increasingly being utilized for the development of biopharmaceuticals, including monoclonal antibody-based drugs, vaccines, and diagnostics. With the rising prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases, pharmaceutical companies are investing heavily in antibody-based therapies. This growing adoption of monoclonal antibodies for diverse applications is driving market growth, as companies seek custom solutions to meet specific therapeutic needs.
Key Trends & Opportunities
Rising Popularity of Antibody-Drug Conjugates (ADCs)
A significant trend in the Monoclonal Antibody Custom Service Market is the rising popularity of antibody-drug conjugates (ADCs). ADCs are a novel class of targeted cancer therapies that combine the specificity of monoclonal antibodies with the potent effects of cytotoxic drugs. This targeted approach minimizes off-target effects and increases the precision of cancer treatment. As ADCs gain traction in clinical trials and approvals, the demand for customized monoclonal antibodies to create these drugs is growing, presenting substantial opportunities for growth in the custom service market.
- For instance, Roche’s development of the ADC Kadcyla, which combines the monoclonal antibody trastuzumab with a cytotoxic agent, has been pivotal in treating HER2-positive breast cancer.
Growing Collaboration Between Research Institutions and Biopharma Companies
A growing trend of collaboration between research institutions and biopharmaceutical companies is opening new opportunities in the Monoclonal Antibody Custom Service Market. Universities, biotech startups, and pharmaceutical giants are increasingly working together to develop cutting-edge antibody-based therapies. These collaborations are fueling demand for custom monoclonal antibody services, as tailored antibodies are required for early-stage research, clinical trials, and eventual product development. This trend is expected to continue, providing opportunities for custom antibody service providers to expand their offerings and capture a larger share of the market.
- For instance, Rockland Immunochemicals collaborates with researchers to provide custom monoclonal antibody production services, optimizing antibodies for challenging targets using their proprietary hybridoma technology for multiple species including mouse and rat.
Key Challenges
High Cost of Custom Monoclonal Antibody Production
One of the significant challenges faced by the Monoclonal Antibody Custom Service Market is the high cost of custom antibody production. The process of developing and manufacturing monoclonal antibodies involves complex techniques, specialized equipment, and significant time investment. These factors contribute to high production costs, making it difficult for smaller biotech firms or research institutions to afford these services. This cost barrier could limit market expansion, especially in emerging markets or for companies with limited budgets for research and development.
Regulatory and Compliance Challenges
Regulatory and compliance challenges represent another hurdle for the Monoclonal Antibody Custom Service Market. Custom monoclonal antibodies must adhere to stringent quality control and regulatory standards to ensure their safety and efficacy in clinical applications. The regulatory approval process for antibody-based therapeutics can be lengthy and complicated, leading to delays in product development and higher costs for custom services. Navigating these regulations and maintaining compliance with ever-evolving standards is a significant challenge for companies providing monoclonal antibody services, potentially hindering growth in the market.
Regional Analysis
North America
The North America region leads the Monoclonal Antibody Custom Service Market with a market share of over 44% in 2022. This dominance stems from a strong ecosystem of pharmaceutical and biotechnology firms, high government funding for life‑science research and robust infrastructure for antibody development. The presence of numerous contract research organisations (CROs) and established academic‑industry collaborations further support service adoption. Increasing demand for personalised therapies and therapeutic antibodies across oncology, immunology and infectious diseases is reinforcing growth in this region.
Europe
Europe commands a significant portion of the monoclonal antibody custom service market, accounting for a share slightly behind North America (estimated in the range of mid‑30% to high‑20% range). Key drivers include well‑established biotechnology hubs in countries such as Germany, the United Kingdom and France, along with supportive regulatory frameworks for antibody development and diagnostics. Increasing investment in biopharmaceutical innovation, the rise of precision medicine and collaborations across European research institutions enhance service demand for custom antibody solutions in this region.
Asia Pacific
The Asia Pacific region is rapidly emerging as a high‑growth market for monoclonal antibody custom services, with a share of 20% in 2022 and anticipated to rise significantly through the forecast period. Growth is driven by increasing healthcare expenditure, expansion of biotech manufacturing capabilities in China, India and South Korea, and a rising prevalence of chronic and infectious diseases. The cost‑advantage of outsourcing and the expansion of local CRO/CMO capacities further support regional uptake of custom monoclonal antibody services.
Latin America & Middle East & Africa
Combined, Latin America along with the Middle East & Africa regions hold the remaining share of the market estimated at around 10% globally. Growth in these areas is gaining momentum due to improving healthcare infrastructure, increasing disease burden and rising interest from global biopharmaceutical companies in outsourcing services. While regulatory and cost‑barrier constraints persist, growing awareness of antibody‑based diagnostics and therapeutics presents emerging opportunities for custom service providers in these markets.
Market Segmentations:
By Product Type
- Rat Custom Monoclonal Antibody
- Rabbit Custom Monoclonal Antibody
- Others
By Application
- Hospitals
- Research & Academic Laboratories
- Pharmaceutical & Biotechnology Companies
- Others
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The competitive landscape in the Monoclonal Antibody Custom Service Market features leading players such as Thermo Fisher Scientific Inc., GenScript, Abcam Plc, Bio‑Rad Laboratories Inc., and Kaneka Eurogentec S.A., among others. These companies actively pursue strategic initiatives such as acquisitions, partnerships, and global facility expansions to strengthen their service offerings and geographic reach. They continue to invest in advanced antibody engineering technologies and scalable production platforms to meet increasing demand from pharmaceutical and biotechnology clients. Smaller niche firms contribute by specialising in customised solutions, thereby raising competitive pressure and driving innovation. As service differentiation becomes increasingly important, providers are emphasising rapid turnaround times, high‑quality validation protocols and value‑added support services to gain an edge in this growing market.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
Recent Developments
- In November 2025, Cell Surface Bio launched VeRSa-Select™, a new custom antibody service designed to deliver the highest quality custom antibodies for validating difficult therapeutic targets.
- In November 2025, Abselion launched a novel assay developed with GenScript’s THE™ His Tag Monoclonal Antibodies, providing researchers a ready-to-use solution for rapid protein quantification, reflecting ongoing product innovation in custom monoclonal antibody services.
- in March 2024, Sino Biological, Inc. announced a collaboration with Rapid Novor, Inc. to leverage Rapid Novor’s REmAb monoclonal antibody sequencing platform aligned with Sino Biological’s custom antibody development and production services, enhancing the pipeline from antibody discovery to delivery.
Report Coverage
The research report offers an in-depth analysis based on Product Type, Application and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will continue to expand as biopharmaceutical companies increase investment in tailored antibody therapies, fueling demand for custom services.
- Growth in personalized medicine will drive the need for highly specific monoclonal antibodies crafted to individual biomarker profiles and rare disease targets.
- Advances in antibody engineering such as recombinant technologies, bispecific formats and improved affinity maturation will reduce development time and open new service avenues.
- The Asia‑Pacific region will emerge as a high‑growth hotspot given rising healthcare expenditure, expanding biotech manufacturing base and increasing outsourcing activity.
- Strategic collaborations between academic institutions, contract research organisations and industry will unlock novel applications and drive service provider growth.
- The rising prevalence of chronic diseases, immunological disorders and oncology indications will create sustained demand for custom monoclonal antibody solutions.
- Service providers will increasingly adopt automation, AI‑driven antibody discovery and high‑throughput platforms to enhance scalability and cost‑efficiency.
- Providers who offer integrated workflows from antigen design through validation and production will gain competitive advantage in a crowded market.
- Regulatory complexity, long development cycles and high‑cost entry will continue to challenge smaller players and limit accessible market entry.
- Market consolidation and provider differentiation will intensify, with key firms forging strategic partnerships and acquisitions to expand geographic reach, capability sets and service portfolios.




