Market Overview:
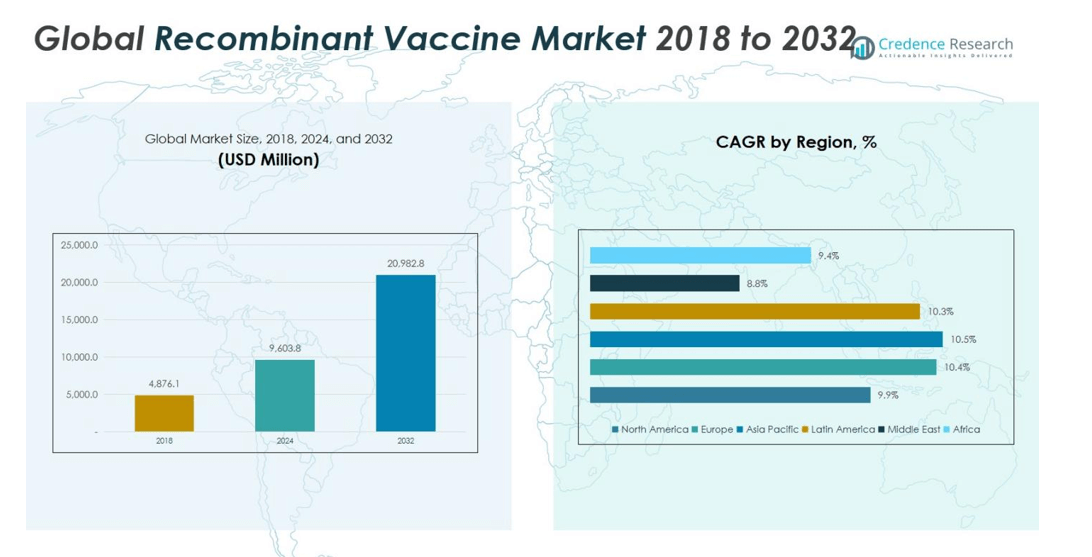
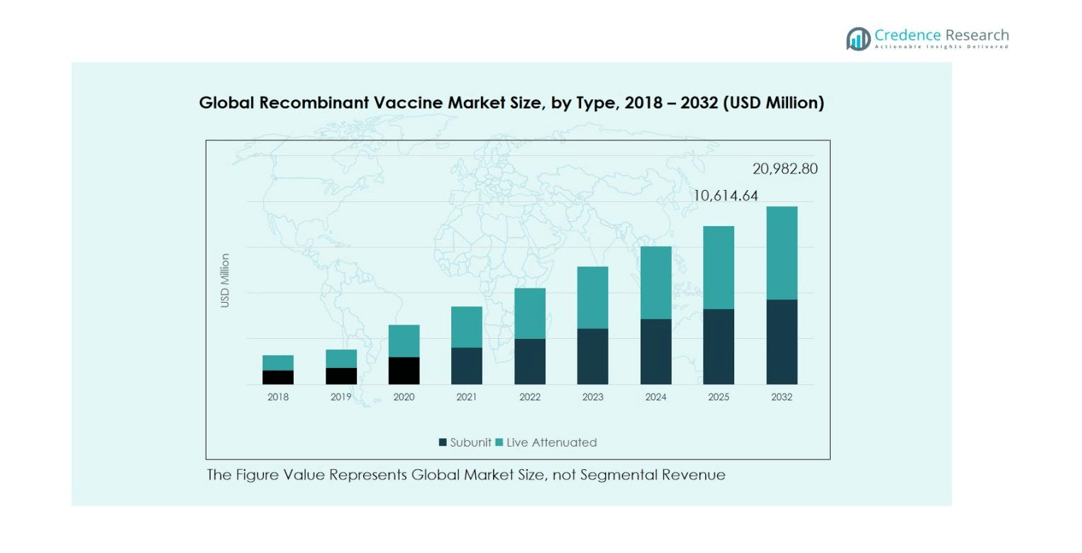
Recombinant Vaccine Market size was valued at USD 4,876.1 Million in 2018, rising to USD 9,603.8 Million in 2024, and is anticipated to reach USD 20,982.8 Million by 2032, at a CAGR of 10.22% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Recombinant Vaccine Market Size 2024 |
USD 9,603.8 Million |
| Recombinant Vaccine Market, CAGR |
10.22% |
| Recombinant Vaccine Market Size 2032 |
USD 20,982.8 Million |
The Recombinant Vaccine Market is dominated by key players such as GSK plc, Serum Institute of India, Novartis AG, Merck & Co., Inc., Pfizer, Inc., Cipla Inc., Sanofi, Bharat Biotech, CanSino Biologics, and AstraZeneca. These companies are driving market growth through strategic collaborations, research and development, and geographic expansion, focusing on innovative vaccine technologies and combination formulations. Asia Pacific leads the global market with a 26% share in 2024, driven by rising population, government immunization programs, and expanding healthcare infrastructure. Europe follows closely, holding 25% of the market, supported by preventive healthcare initiatives and strong regulatory frameworks. North America, with 23% market share, benefits from advanced R&D, high adoption rates, and extensive vaccination campaigns. Collectively, these regions account for the majority of global market revenue, while emerging markets in Latin America, the Middle East, and Africa offer significant growth opportunities for the leading players to expand their portfolios and reach underserved populations.
Market Insights
- The Recombinant Vaccine Market was valued at USD 9,603.8 Million in 2024 and is projected to reach USD 20,982.8 Million by 2032, growing at a CAGR of 10.22%. Subunit vaccines hold 62% of the market share in 2024, and parenteral administration dominates with 78% share, while Hepatitis B vaccines lead disease indications with 28% share.
- Rising prevalence of infectious diseases, increasing government immunization programs, and growing awareness about vaccine-preventable illnesses are driving the market globally.
- Market trends include the growing adoption of combination and multivalent vaccines, technological advancements in recombinant DNA vaccines, and expansion into emerging regions such as Asia Pacific and Latin America.
- The market is competitive, with key players including GSK plc, Serum Institute of India, Novartis AG, Merck & Co., Pfizer, Sanofi, Bharat Biotech, CanSino Biologics, and AstraZeneca, focusing on R&D, strategic collaborations, and geographic expansion to strengthen their position.
- High manufacturing costs, complex production processes, and stringent regulatory requirements act as restraints, while North America, Europe, and Asia Pacific together account for the majority of market revenue.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Segmentation Analysis:
By Type:
The recombinant vaccine market by type is primarily segmented into subunit and live attenuated vaccines. Subunit vaccines dominate the market, holding 62% of the share in 2024, driven by their safety profile, ability to target specific antigens, and lower risk of adverse reactions compared to live attenuated vaccines. The rising prevalence of chronic infectious diseases and growing adoption in pediatric and adult immunization programs further support their growth. Live attenuated vaccines, while effective, face limited uptake due to cold chain requirements and potential safety concerns in immunocompromised populations.
- For instance, the Baiya SARS-CoV-2 Vax 1 subunit vaccine demonstrated strong neutralizing antibody responses in preclinical trials using low antigen doses, highlighting the efficiency and adaptability of subunit vaccine formulations.
By Route of Administration:
Recombinant vaccines are administered via parenteral and oral routes, with parenteral vaccines capturing 78% of the market share in 2024. Their dominance is fueled by higher efficacy, broader clinical acceptance, and established injection-based immunization programs across hospitals and clinics globally. Oral vaccines, although convenient and needle-free, account for a smaller share due to stability issues and limited indications. Increasing government immunization initiatives and growing awareness about vaccine-preventable diseases are driving the overall market, reinforcing the preference for parenteral administration in routine and mass vaccination campaigns.
- For instance, the COVID-19 vaccine Convidecia Air®, an inhaled recombinant vaccine developed by CanSinoBIO, exemplifies the growth in nasal delivery methods, leveraging inhalation to stimulate mucosal immunity effectively.
By Disease Indication:
Within disease indications, the market is led by Hepatitis B vaccines, holding 28% of the market share in 2024, driven by mandatory immunization programs and rising prevalence of Hepatitis B globally. Human Papillomavirus (HPV) vaccines follow closely, supported by cervical cancer prevention initiatives. Rotavirus, herpes zoster, and meningococcal B vaccines contribute smaller shares but are witnessing growth due to increasing pediatric vaccination programs and awareness of adult immunization. The market growth is propelled by government-led vaccination campaigns, expanding healthcare infrastructure, and rising focus on disease prevention and immunization coverage worldwide.
Key Growth Drivers
Rapid Rise in Infectious Diseases
The increasing prevalence of infectious diseases worldwide acts as a key growth driver for the recombinant vaccine market. Governments and healthcare organizations are intensifying vaccination programs to control diseases such as Hepatitis B, HPV, and Rotavirus. Rising awareness about disease prevention, coupled with growing public health initiatives, is boosting vaccine adoption. Additionally, urbanization, global travel, and changing lifestyle patterns contribute to higher exposure to pathogens, increasing the demand for safe and effective recombinant vaccines across all age groups, thereby driving market expansion.
- For instance, the quadrivalent HPV vaccine showed 100% efficacy against four HPV subtypes (6/11/16/18) three years post-vaccination, significantly reducing the incidence of cervical lesions in young women.
Technological Advancements in Vaccine Development
Continuous innovation in recombinant DNA technology and vaccine formulations is propelling market growth. Advanced techniques allow the development of subunit and multivalent vaccines that are highly specific, safer, and effective against complex infectious agents. Improvements in adjuvants, delivery systems, and stability of vaccines enhance immunogenicity and shelf life. These technological developments not only increase the acceptance of recombinant vaccines among healthcare providers and patients but also expand applications across new disease indications, strengthening overall market adoption and facilitating faster response to emerging infectious diseases.
- For instance, advanced adjuvants like AS37, a synthetic TLR7a agonist adjuvant combined with alum, have shown potent immunogenicity and acceptable safety profiles in Phase I clinical trials for meningococcal and hepatitis B vaccines.
Government Initiatives and Immunization Programs
Supportive government policies and large-scale immunization programs worldwide are significantly driving the recombinant vaccine market. Initiatives by agencies such as WHO, CDC, and national healthcare authorities promote vaccination awareness and accessibility, particularly in developing regions. Subsidized vaccine programs, mandatory immunization schedules, and public-private collaborations encourage higher uptake. Funding for research and development, coupled with policies ensuring affordability and distribution, reinforces vaccine penetration, enabling wider population coverage. Such government-led efforts create a favorable regulatory environment, boosting both market growth and public trust in recombinant vaccines.
Key Trends and Opportunities
Rising Adoption of Combination and Multivalent Vaccines
A prominent trend in the recombinant vaccine market is the growing preference for combination and multivalent vaccines, which protect against multiple pathogens in a single dose. This approach reduces the number of injections required, improves patient compliance, and streamlines immunization schedules. Pediatric and adult immunization programs increasingly favor these vaccines to enhance coverage and efficiency. Manufacturers are investing in developing innovative formulations that combine antigens without compromising safety or efficacy, creating significant market opportunities in regions with high vaccination demand and limited healthcare resources.
- For instance, Sanofi Pasteur developed Hexyon, a fully-liquid hexavalent combination vaccine protecting against diphtheria, tetanus, pertussis, poliovirus, Hib, and hepatitis B, streamlining vaccination schedules.
Expansion into Emerging Markets
Emerging markets in Asia Pacific, Latin America, and Africa present substantial growth opportunities for recombinant vaccines due to rising healthcare awareness, expanding vaccination infrastructure, and increasing disposable incomes. Governments in these regions are prioritizing immunization programs to combat infectious diseases, creating demand for reliable vaccine solutions. Local manufacturing, strategic partnerships, and technology transfers further facilitate market penetration. This expansion allows global players to tap into underserved populations while addressing regional healthcare challenges, driving revenue growth and fostering long-term sustainability in the recombinant vaccine sector.
- For instance, Sinergium Biotech in Argentina and BioManguinhos in Brazil are actively participating in mRNA vaccine technology transfer programs, supporting local development to reduce import dependency and improve timely vaccine access.
Key Challenges
High Manufacturing Costs and Complex Production
Recombinant vaccines involve intricate production processes, including advanced cell culture systems and stringent quality control, leading to high manufacturing costs. These factors limit affordability and accessibility, particularly in low-income regions. Production complexity also increases dependency on specialized facilities and skilled personnel, constraining rapid scale-up during outbreaks. Companies must invest heavily in R&D and infrastructure to maintain consistent supply and regulatory compliance. These challenges can impact market growth by restricting adoption rates, particularly where cost-sensitive healthcare systems struggle to implement large-scale vaccination programs.
Regulatory and Safety Concerns
Strict regulatory frameworks and stringent approval processes pose a significant challenge for recombinant vaccines. Regulatory agencies require extensive clinical trials to validate safety and efficacy, which can delay product launches and increase development costs. Additionally, public concerns about adverse effects and vaccine hesitancy can affect market acceptance. Compliance with international standards and post-marketing surveillance further complicates commercialization. These regulatory and safety hurdles necessitate continuous investment in quality assurance and monitoring, slowing market expansion despite growing global demand for recombinant vaccines.
Regional Analysis
North America
North America’s recombinant vaccine market was valued at USD 1,154.18 million in 2018, increasing to USD 2,232.07 million in 2024, and is projected to reach USD 4,756.80 million by 2032, at a CAGR of 9.9%. The region holds 23% of the global market share in 2024, driven by strong healthcare infrastructure, government immunization programs, and high awareness of vaccine-preventable diseases. The U.S. dominates the market through advanced R&D, innovative vaccines, and strategic collaborations, ensuring consistent adoption and growth.
Europe
Europe was valued at USD 1,289.74 million in 2018, is expected to reach USD 2,571.09 million in 2024, and is projected at USD 5,707.32 million by 2032, growing at a CAGR of 10.4%. With a 25% market share in 2024, strong healthcare infrastructure, preventive vaccination programs, and strict regulatory frameworks drive growth. Key countries such as the UK, Germany, and France lead adoption through government campaigns and investments in vaccine technology, particularly for HPV, Hepatitis B, and Rotavirus.
Asia Pacific
Asia Pacific’s market was valued at USD 1,566.22 million in 2018, projected to reach USD 3,131.68 million in 2024, and estimated at USD 6,978.88 million by 2032, registering a CAGR of 10.5%. Accounting for 26% of the global market share in 2024, the region benefits from rising population, improving healthcare infrastructure, and government vaccination initiatives. China, India, and Japan drive growth through local production, affordability programs, and expanding pediatric and adult immunization programs.
Latin America
Latin America’s recombinant vaccine market was USD 525.65 million in 2018, expected to grow to USD 1,039.82 million in 2024, and projected at USD 2,285.03 million by 2032, at a CAGR of 10.3%. Holding around 11% of the global market share in 2024, Brazil and Argentina lead adoption through government vaccination programs, local manufacturing, and partnerships with global vaccine makers. Rising healthcare access and awareness campaigns are boosting market growth across the region.
Middle East
The Middle East market was valued at USD 233.08 million in 2018, rising to USD 426.96 million in 2024, and expected to reach USD 839.31 million by 2032, growing at a CAGR of 8.8%. With around 4% of the global market share in 2024, growth is driven by government vaccination programs, expanding healthcare infrastructure, and partnerships with global manufacturers. GCC countries, Israel, and Turkey dominate the market, supported by increasing awareness of infectious disease prevention.
Africa
Africa’s recombinant vaccine market stood at USD 107.28 million in 2018, projected to reach USD 202.23 million in 2024, and expected to grow to USD 415.46 million by 2032, with a CAGR of 9.4%. Representing 2% of the global market share in 2024, growth is supported by immunization programs, international funding, and healthcare partnerships. South Africa and Egypt are key contributors, focusing on HPV, Hepatitis B, and Rotavirus vaccines to expand access and improve vaccination coverage.
Market Segmentations:
By Type
By Route of Administration
By Disease Indication
- Human Papillomavirus (HPV)
- Hepatitis B
- Rotavirus
- Herpes Zoster
- Meningococcal B
- Others
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The competitive landscape of the recombinant vaccine market is dominated by key players such as GSK plc, Serum Institute of India, Novartis AG, Merck & Co., Inc., Pfizer, Inc., Cipla Inc., Sanofi, Bharat Biotech, CanSino Biologics, and AstraZeneca. These companies are actively focusing on research and development, strategic collaborations, and product innovation to strengthen their market position. The market is characterized by high entry barriers due to complex regulatory approvals and advanced manufacturing requirements, giving established players a competitive advantage. Companies are expanding their portfolios through acquisitions, partnerships, and geographic expansion to capture emerging markets, particularly in Asia Pacific and Latin America. Continuous investment in novel vaccine technologies, combination and multivalent vaccines, and improvements in delivery systems further enhance competitiveness. Additionally, strong government collaborations and large-scale immunization programs support market penetration and long-term growth, making strategic innovation and distribution efficiency critical success factors.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- GSK plc
- Serum Institute of India
- Novartis AG
- Merck & Co., Inc.
- Pfizer, Inc.
- Cipla Inc.
- Sanofi
- Bharat Biotech
- CanSino Biologics
- AstraZeneca
- Other Key Players
Recent Developments
- In May 2024, Novavax, Inc. entered into a co-exclusive licensing agreement with Sanofi S.A. to co-commercialize its adjuvanted COVID-19 recombinant protein vaccine globally, excluding certain Asia-Pacific markets.
- In April 2025, GC Biopharma gained approval from South Korea’s Ministry of Food and Drug Safety (MFDS) for BARYTHRAX, recognized as the world’s first recombinant anthrax vaccine.
- In March 2025, ImmunityBio, Inc. began the first dosing of its recombinant BCG vaccine (rBCG) under the Expanded Access Program (EAP) in the United States to help address BCG shortages used in bladder cancer treatment.
- In July 2025, Vaxart, Inc. announced that its stock began trading on OTCQX and highlighted the continued development of its oral recombinant pill-based vaccines targeting coronavirus, norovirus, and influenza, using its proprietary vaccine platform.
Report Coverage
The research report offers an in-depth analysis based on Type, Route of Administration, Disease Indication and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market is expected to grow steadily due to increasing prevalence of infectious diseases worldwide.
- Rising government immunization programs will continue to boost vaccine adoption across regions.
- Technological advancements in recombinant DNA and vaccine formulations will drive innovation and effectiveness.
- Growing preference for combination and multivalent vaccines will enhance patient compliance and coverage.
- Expansion into emerging markets in Asia Pacific, Latin America, and Africa will provide significant growth opportunities.
- Pediatric and adult immunization programs will further strengthen demand for recombinant vaccines.
- Strategic partnerships and collaborations among key players will accelerate product development and market reach.
- Increasing awareness and focus on disease prevention will support market growth.
- Regulatory support and streamlined approval processes will facilitate faster market entry of novel vaccines.
- Investments in manufacturing infrastructure and distribution channels will improve accessibility and scalability globally.






