Chapter No. 1 : Introduction 35
1.1. Report Description 35
1.1.1. Purpose of the Report 35
1.1.2. USP & Key Offerings 35
1.2. Key Benefits for Stakeholders 35
1.3. Target Audience 36
1.4. Report Scope 37
Chapter No. 2 : Executive Summary 39
2.1. Pharma R&D Outsourcing Market Snapshot 39
2.2. U.S. Pharma R&D Outsourcing Market, 2017 – 2022 (USD Million) 40
Chapter No. 3 : Impact Analysis of COVID 19 & Russia-Ukraine War on Pharma R&D Outsourcing Market 41
3.1. Impact Assessment of COVID-19 Pandemic, By Region 41
3.1.1. Impact of COVID-19 on U.S. Pharma R&D Outsourcing: 41
3.1.2. Geopolitical Impact on U.S. Pharma R&D Outsourcing: 42
3.1.3. Israel’s Role in Pharma R&D: 43
3.1.4. Considerations for the Future: 43
Chapter No. 4 : Pharma R&D Outsourcing Market – Industry Analysis 44
4.1. Introduction 44
4.2. Market Drivers 45
4.2.1. Numerous benefits arise from outsourcing pharmaceutical research and development 45
4.2.2. Rising Outsourcing of Research and Development 46
4.2.3. Increased Demand for Outsourced Services 46
4.3. Market Restraints 47
4.3.1. Clinical Trial Failures 47
4.3.2. Stringent Government Regulations 48
4.4. Market Opportunities 49
4.4.1. Emergence of Pharma R&D Outsourcing in Developing Countries 49
4.4.2. Increased R&D by Biopharmaceutical Vendors 50
4.5. Porter’s Five Forces Analysis 51
4.6. Value Chain Analysis 53
4.7. Buying Criteria 56
Chapter No. 5 : Analysis Competitive Landscape 57
5.1. Company Market Share Analysis – 2022 57
5.1.1. U.S. Pharma R&D Outsourcing Market: Top 5 Company Market Share, by Revenue 2022 57
5.1.2. U.S. Pharma R&D Outsourcing Market: Top 10 Company Market Share, by Revenue 2022 58
5.1. U.S. Pharma R&D Outsourcing Market Company Revenue Market Share, 2022 59
5.2. Company Assessment Metrics 60
5.2.1. Stars 60
5.2.2. Emerging Leaders 60
5.2.3. Pervasive Players 60
5.2.4. Participants 60
5.3. Key Players Product Matrix 61
Chapter No. 6 : PESTEL & Adjacent Market Analysis 65
6.1. PESTEL 65
6.1.1. Political Factors 65
6.1.2. Economic Factors 65
6.1.3. Social Factors 65
6.1.4. Technological Factors 65
6.1.5. Environmental Factors 66
6.1.6. Legal Factors 66
6.2. Adjacent Market Analysis 67
Chapter No. 7 : Pharma R&D Outsourcing Market – By Type Segment Analysis 70
7.1. Pharma R&D Outsourcing Market Overview, by Type Segment 70
7.1.1. Pharma R&D Outsourcing Market Revenue Share, By Type, 2022 & 2030 70
7.1.2. Pharma R&D Outsourcing Market Attractiveness Analysis, By Type 71
7.1.3. Incremental Revenue Growth Opportunity, by Type, 2023 – 2030 71
7.1.4. Pharma R&D Outsourcing Market Revenue, By Type, 2017 to 2022 72
7.2. Small Molecules 73
7.3. Biologics 74
Chapter No. 8 : Pharma R&D Outsourcing Market – By Therapy Area Segment Analysis 75
8.1. Pharma R&D Outsourcing Market Overview, by Therapy Area Segment 75
8.1.1. Pharma R&D Outsourcing Market Revenue Share, By Therapy Area, 2022 & 2030 75
8.1.2. Pharma R&D Outsourcing Market Attractiveness Analysis, By Therapy Area 76
8.1.3. Incremental Revenue Growth Opportunity, by Therapy Area, 2023 – 2030 76
8.1.4. Pharma R&D Outsourcing Market Revenue, By Therapy Area, 2017 to 2022 77
8.2. Oncology 78
8.3. Cardiovascular Diseases 79
8.4. Infectious Diseases 80
8.5. Musculoskeletal Disorders 81
8.6. Others 82
Chapter No. 9 : Pharma R&D Outsourcing Market – By End-user Segment Analysis 83
9.1. Pharma R&D Outsourcing Market Overview, by End-user Segment 83
9.1.1. Pharma R&D Outsourcing Market Revenue Share, By End-user, 2022 & 2030 83
9.1.2. Pharma R&D Outsourcing Market Attractiveness Analysis, By End-user 84
9.1.3. Incremental Revenue Growth Opportunity, by End-user, 2023 – 2030 84
9.1.4. Pharma R&D Outsourcing Market Revenue, By End-user, 2017 to 2022 85
9.2. Small & Mid-Sized Companies 86
9.3. Large Companies 87
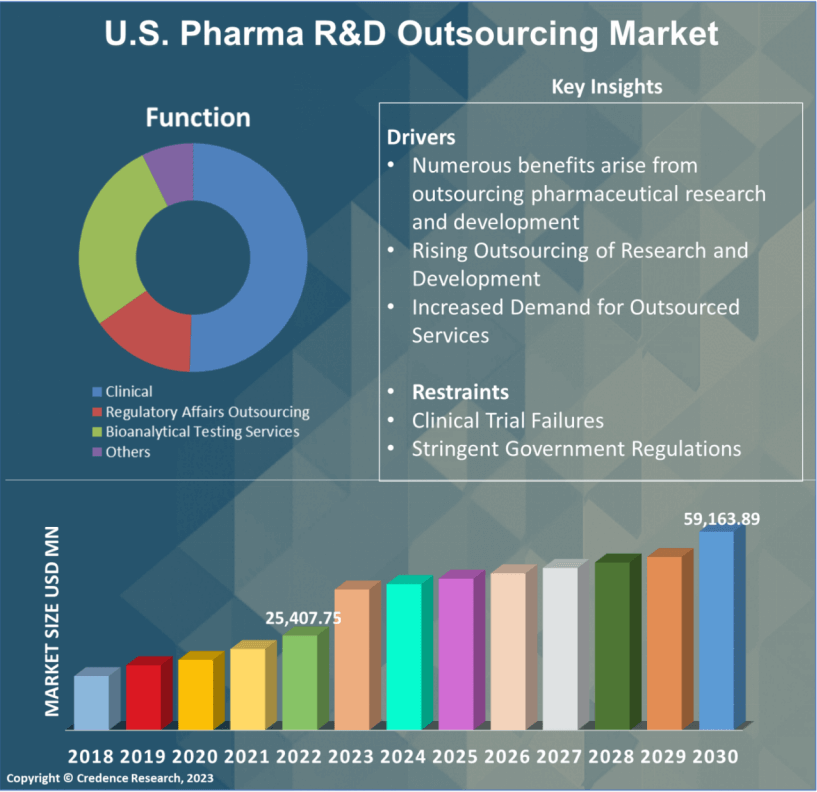
Chapter No. 10 : Pharma R&D Outsourcing Market – By Function Segment Analysis 88
10.1. Pharma R&D Outsourcing Market Overview, by Function Segment 88
10.1.1. Pharma R&D Outsourcing Market Revenue Share, By Function, 2022 & 2030 88
10.1.2. Pharma R&D Outsourcing Market Attractiveness Analysis, By Function 89
10.1.3. Incremental Revenue Growth Opportunity, by Function, 2023 – 2030 90
10.1.4. Pharma R&D Outsourcing Market Revenue, By Function, 2017 to 2022 91
10.2. Clinical 92
10.3. Regulatory Affairs Outsourcing 93
10.4. Bioanalytical Testing Services 94
10.5. Others 95
Chapter No. 11 : Company Profiles 96
11.1. Charles River Laboratories 96
11.1.1. Company Overview 96
11.1.2. Product Portfolio 97
11.1.3. Financial Overview 97
11.2. ICON 98
11.2.1. Company Overview 98
11.2.2. Product Portfolio 99
11.2.3. Financial Overview 99
11.3. IQVIA 100
11.3.1. Company Overview 100
11.3.2. Product Portfolio 101
11.3.3. Financial Overview 101
11.4. Labcorp Drug Development 102
11.4.1. Company Overview 102
11.4.2. 102
11.4.3. Product Portfolio 103
11.4.4. Financial Overview 103
11.5. Medpace 104
11.5.1. Company Overview 104
11.5.2. Product Portfolio 105
11.5.3. Financial Overview 105
11.6. Parexel International Corporation 106
11.6.1. Company Overview 106
11.6.2. Product Portfolio 107
11.6.3. Financial Overview 107
11.7. Syneos Health 108
11.7.1. Company Overview 108
11.7.2. Product Portfolio 108
11.7.3. Financial Overview 109
11.8. Thermo Fisher Scientific 110
11.8.1. Company Overview 110
11.8.2. Product Portfolio 111
11.8.3. Financial Overview 111
11.9. Wuxi AppTec 112
11.9.1. Company Overview 112
11.9.2. Product Portfolio 113
11.9.3. Financial Overview 113
11.10. Lonza 114
11.10.1. Company Overview 114
11.10.2. Product Portfolio 115
11.10.3. Financial Overview 115
11.11. Boehringer Ingelheim 116
11.11.1. Company Overview 116
11.11.2. Product Portfolio 116
11.11.3. Financial Overview 117
11.12. Samsung Biologics 118
11.12.1. Company Overview 118
11.12.2. Product Portfolio 119
11.12.3. Financial Overview 119
11.13. AbbVie 120
11.13.1. Company Overview 120
11.13.2. Product Portfolio 121
11.13.3. Financial Overview 121
11.14. Advanced Clinical 122
11.14.1. Company Overview 122
11.14.2. Product Portfolio 122
11.14.3. Financial Overview 123
11.15. BioAnalytix 124
11.15.1. Company Overview 124
11.15.2. Product Portfolio 125
11.15.3. Financial Overview 125
11.16. Asymchem 126
11.16.1. Company Overview 126
11.16.2. Product Portfolio 127
11.16.3. Financial Overview 127
11.17. Alcami 128
11.17.1. Company Overview 128
11.17.2. Product Portfolio 128
11.17.3. Financial Overview 129
11.18. Catalent 130
11.18.1. Company Overview 130
11.18.2. Product Portfolio 131
11.18.3. Financial Overview 131
11.19. Curia U.S. 132
11.19.1. Company Overview 132
11.19.2. Product Portfolio 132
11.19.3. Financial Overview 133
11.20. ChemPartner 134
11.20.1. Company Overview 134
11.20.2. Product Portfolio 135
11.20.3. Financial Overview 135
11.21. CMED 136
11.21.1. Company Overview 136
11.21.2. Product Portfolio 136
11.21.3. Financial Overview 137
11.22. Criterium 138
11.22.1. Company Overview 138
11.22.2. Product Portfolio 139
11.22.3. Financial Overview 139
11.23. Cromos Pharma 140
11.23.1. Company Overview 140
11.23.2. Product Portfolio 141
11.23.3. Financial Overview 141
11.24. Evotec 142
11.24.1. Company Overview 142
11.24.2. Product Portfolio 143
11.24.3. Financial Overview 143
11.25. KBI Biopharma 144
11.25.1. Company Overview 144
11.25.2. Product Portfolio 144
11.25.3. Financial Overview 145
11.26. KCR S.A. 146
11.26.1. Company Overview 146
11.26.2. Product Portfolio 147
11.26.3. Financial Overview 147
11.27. Kemwell Biopharma 148
11.27.1. Company Overview 148
11.27.2. Product Portfolio 149
11.27.3. Financial Overview 149
11.28. Mesned Pharma Consult Center 150
11.28.1. Company Overview 150
11.28.2. Product Portfolio 150
11.28.3. Financial Overview 151
11.29. Medelis 152
11.29.1. Company Overview 152
11.29.2. Product Portfolio 152
11.29.3. Financial Overview 153
11.30. OCT Clinical 154
11.30.1. Company Overview 154
11.30.2. Product Portfolio 155
11.30.3. Financial Overview 155
11.31. ProTrials Research 156
11.31.1. Company Overview 156
11.31.2. Product Portfolio 157
11.31.3. Financial Overview 157
11.32. PROMETRIKA 158
11.32.1. Company Overview 158
11.32.2. Product Portfolio 158
11.32.3. Financial Overview 159
11.33. QPS 160
11.33.1. Company Overview 160
11.33.2. Product Portfolio 161
11.33.3. Financial Overview 161
11.34. Singota Solutions 162
11.34.1. Company Overview 162
11.34.2. Product Portfolio 163
11.34.3. Financial Overview 163
11.35. Sofpromed 164
11.35.1. Company Overview 164
11.35.2. Product Portfolio 164
11.35.3. Financial Overview 165
11.36. WuXi Biologics 166
11.36.1. Company Overview 166
11.36.2. Product Portfolio 167
11.36.3. Financial Overview 167
Chapter No. 12 : Research Methodology 168
12.1. Research Methodology 168
12.2. Phase I – Secondary Research 169
12.3. Phase II – Data Modeling 169
12.3.1. Company Share Analysis Model 170
12.3.2. Revenue Based Modeling 170
12.4. Phase III – Primary Research 171
12.5. Research Limitations 172
12.5.1. Assumptions 172
List of Figures
FIG NO. 1. U.S. Pharma R&D Outsourcing Market Revenue, 2017 – 2022 (USD Million) 40
FIG NO. 2. Comparison of Research Costs in US and India 46
FIG NO. 3. Key Factors in Failure of Clinical Trials 47
FIG NO. 4. Porter’s Five Forces Analysis for U.S. Pharma R&D Outsourcing Market 51
FIG NO. 5. Company Share Analysis, 2022 57
FIG NO. 6. Company Share Analysis, 2022 58
FIG NO. 7. Pharma R&D Outsourcing Market – Company Revenue Market Share, 2022 59
FIG NO. 8. Company Assessment Metrics 60
FIG NO. 9. Pharma R&D Outsourcing Market Revenue Share, By Type, 2022 & 2030 70
FIG NO. 10. Market Attractiveness Analysis, By Type 71
FIG NO. 11. Incremental Revenue Growth Opportunity by Type 71
FIG NO. 12. Pharma R&D Outsourcing Market Revenue, By Type, 2017 to 2022 72
FIG NO. 13. U.S. Pharma R&D Outsourcing Market for Small Molecules, Revenue (USD Million) 2017 – 2030 73
FIG NO. 14. U.S. Pharma R&D Outsourcing Market for Biologics, Revenue (USD Million) 2017 – 2030 74
FIG NO. 15. Pharma R&D Outsourcing Market Revenue Share, By Therapy Area, 2022 & 2030 75
FIG NO. 16. Market Attractiveness Analysis, By Therapy Area 76
FIG NO. 17. Incremental Revenue Growth Opportunity by Therapy Area 76
FIG NO. 18. Pharma R&D Outsourcing Market Revenue, By Therapy Area, 2017 to 2022 77
FIG NO. 19. U.S. Pharma R&D Outsourcing Market for Oncology, Revenue (USD Million) 2017 – 2030 78
FIG NO. 20. U.S. Pharma R&D Outsourcing Market for Cardiovascular Diseases, Revenue (USD Million) 2017 – 2030 79
FIG NO. 21. U.S. Pharma R&D Outsourcing Market for Infectious Diseases, Revenue (USD Million) 2017 – 2030 80
FIG NO. 22. U.S. Pharma R&D Outsourcing Market for Musculoskeletal Disorders, Revenue (USD Million) 2017 – 2030 81
FIG NO. 23. U.S. Pharma R&D Outsourcing Market for Others, Revenue (USD Million) 2017 – 2030 82
FIG NO. 24. Pharma R&D Outsourcing Market Revenue Share, By End-user, 2022 & 2030 83
FIG NO. 25. Market Attractiveness Analysis, By End-user 84
FIG NO. 26. Incremental Revenue Growth Opportunity by End-user 84
FIG NO. 27. Pharma R&D Outsourcing Market Revenue, By End-user, 2017 to 2022 85
FIG NO. 28. U.S. Pharma R&D Outsourcing Market for Small & Mid-Sized Companies, Revenue (USD Million) 2017 – 2030 86
FIG NO. 29. U.S. Pharma R&D Outsourcing Market for Large Companies, Revenue (USD Million) 2017 – 2030 87
FIG NO. 30. Pharma R&D Outsourcing Market Revenue Share, By Function, 2022 & 2030 88
FIG NO. 31. Market Attractiveness Analysis, By Function 89
FIG NO. 32. Incremental Revenue Growth Opportunity by Function 90
FIG NO. 33. Pharma R&D Outsourcing Market Revenue, By Function, 2017 to 2022 91
FIG NO. 34. U.S. Pharma R&D Outsourcing Market for Clinical, Revenue (USD Million) 2017 – 2030 92
FIG NO. 35. U.S. Pharma R&D Outsourcing Market for Regulatory Affairs Outsourcing, Revenue (USD Million) 2017 – 2030 93
FIG NO. 36. U.S. Pharma R&D Outsourcing Market for Bioanalytical Testing Services, Revenue (USD Million) 2017 – 2030 94
FIG NO. 37. U.S. Pharma R&D Outsourcing Market for Others (Euglena, etc.), Revenue (USD Million) 2017 – 2030 95
FIG NO. 38. Research Methodology – Detailed View 168
FIG NO. 39. Research Methodology 169
List of Tables
TABLE NO. 1. : U.S. Pharma R&D Outsourcing Market: Snapshot 23
TABLE NO. 2. : Drivers for the Pharma R&D Outsourcing Market: Impact Analysis 29
TABLE NO. 3. : Restraints for the Pharma R&D Outsourcing Market: Impact Analysis 31