Market Overview:
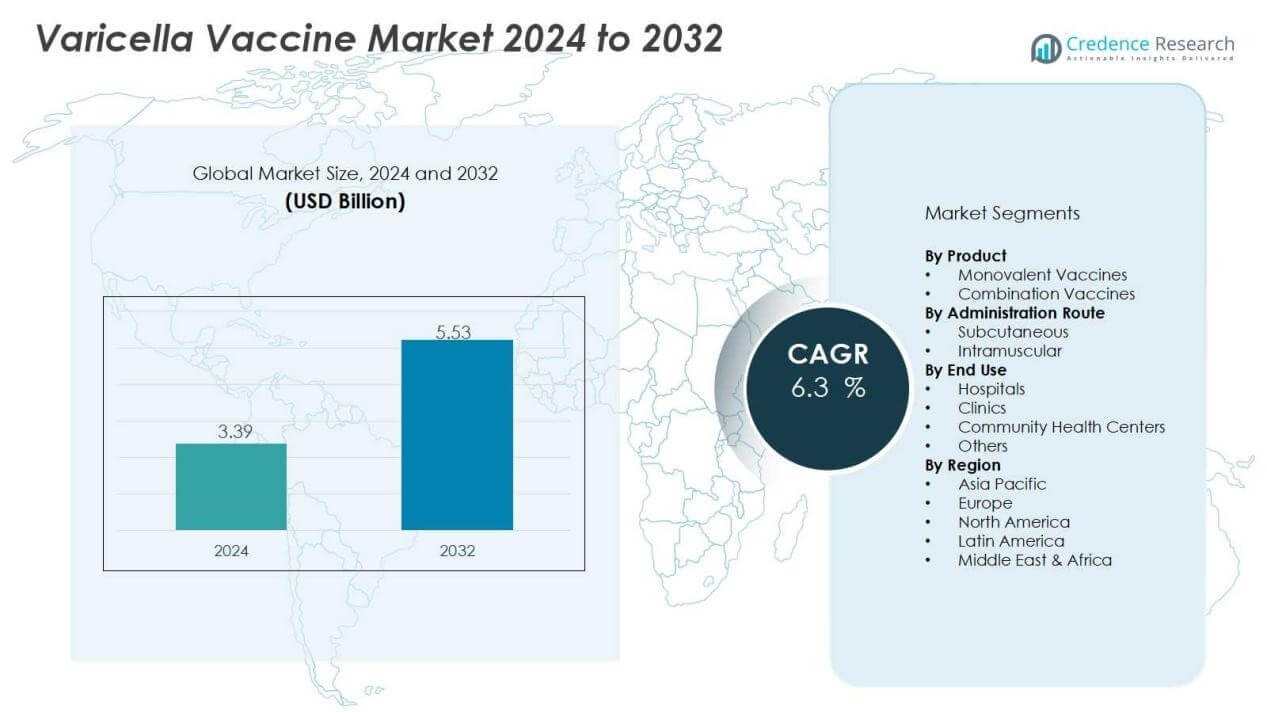
The varicella vaccine market size was valued at USD 3.39 billion in 2024 and is anticipated to reach USD 5.53 billion by 2032, at a CAGR of 6.3 % during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Varicella Vaccine MarketSize 2024 |
USD 3.39 Billion |
| Varicella Vaccine Market, CAGR |
6.3 % |
| Varicella Vaccine Market Size 2032 |
USD 5.53 Billion |
Strong market drivers include growing awareness of immunization benefits, reduced hospitalization costs, and rising prevalence of chickenpox in regions with low coverage. The introduction of combination vaccines, along with WHO and CDC recommendations, enhances adoption. Increasing investment in vaccine R&D and collaborations between pharmaceutical companies and public health bodies further accelerate market expansion.
Regionally, North America dominates the varicella vaccine market due to robust healthcare infrastructure and widespread immunization programs. Europe follows with strong uptake driven by government-backed vaccination campaigns and stringent health policies. The Asia-Pacific region is projected to register the fastest growth, fueled by large pediatric populations, expanding healthcare expenditure, and improved vaccine distribution networks. Meanwhile, Latin America and the Middle East & Africa show emerging opportunities as vaccination awareness and access improve.
 Market Insights:
Market Insights:
- The varicella vaccine market was valued at USD 3.39 billion in 2024 and is projected to reach USD 5.53 billion by 2032, growing at a CAGR of 6.3%.
- Growing awareness of immunization benefits, reduced hospitalization costs, and rising prevalence of chickenpox in low-coverage regions are key drivers.
- WHO and CDC recommendations, along with the introduction of combination vaccines, continue to strengthen global adoption.
- Investments in R&D and collaborations between pharmaceutical companies and public health bodies create opportunities for next-generation vaccines.
- High vaccination costs, limited access in low-income regions, and cold-chain logistics challenges hinder adoption.
- Vaccine hesitancy and misconceptions about safety remain significant barriers, slowing uptake in vulnerable populations.
- North America led with 41% share in 2024, Europe followed with 28%, and Asia-Pacific secured 21%, with the fastest growth ahead.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Focus on Preventive Healthcare and Immunization Programs:
The varicella vaccine market benefits from a growing emphasis on preventive healthcare. Governments and healthcare organizations prioritize vaccination to reduce the clinical and economic burden of chickenpox. National immunization schedules increasingly include varicella vaccines to expand coverage. This widespread adoption continues to strengthen demand and reduce outbreaks.
- For instance, SK bioscience’s SKYVaricella® achieved WHO prequalification in December 2019, marking it as only the second varicella vaccine globally to receive this recognition, thereby expanding access to over 150 countries.
Strong Government Support and Global Health Recommendations:
Public health agencies such as the World Health Organization and the Centers for Disease Control endorse the varicella vaccine. These recommendations encourage countries to integrate the vaccine into routine immunization. Government subsidies and large-scale vaccination drives help improve access. It supports higher uptake rates, particularly in regions with developing healthcare systems.
- For instance, in Japan, the Biken Institute’s live attenuated varicella vaccine, administered to 1.39 million people between 1987 and 1993, achieved a seroconversion rate of 91.5% in tested patients, demonstrating strong long-term protection across more than 20 years of follow-up.
Increasing R&D and Pharmaceutical Collaborations:
The varicella vaccine market gains momentum from continuous research and development. Pharmaceutical companies invest in improving vaccine formulations, stability, and efficacy. Collaborations between manufacturers and healthcare authorities help accelerate clinical trials and distribution. It creates opportunities for next-generation vaccines and strengthens global supply.
Expanding Pediatric Population and Rising Awareness Levels:
A growing pediatric population drives significant demand for varicella immunization. Parents and healthcare providers recognize the importance of protecting children from complications linked to chickenpox. Awareness campaigns highlight vaccine safety and effectiveness, which fosters public confidence. It reinforces long-term adoption trends across both developed and emerging regions.
Market Trends:
Growing Adoption of Combination Vaccines and Technological Advancements:
The varicella vaccine market is witnessing rising demand for combination vaccines that protect against multiple diseases in a single dose. This trend helps reduce the number of injections, improve compliance, and optimize healthcare resources. Pharmaceutical companies focus on advancing formulations to enhance stability, shelf life, and overall efficacy. It reflects an industry-wide effort to meet global immunization goals while minimizing logistical challenges. Digital tracking tools and improved cold-chain technologies are also becoming vital to ensure proper storage and distribution. Together, these innovations contribute to higher efficiency across supply chains and broader vaccine availability.
- For Instance,Merck’s ProQuad vaccine administered to over 31,000 children revealed a higher rate of fever and febrile seizures in children aged 12 to 23 months after the first dose compared to separate vaccinations.
Expanding Global Immunization Coverage and Increasing Private Sector Involvement:
The varicella vaccine market benefits from wider inclusion in routine immunization programs across both developed and emerging economies. Governments promote mass vaccination campaigns, supported by international organizations that aim to reduce preventable diseases. It strengthens healthcare infrastructure and builds resilience against disease outbreaks. Private sector participation, particularly from pharmaceutical and biotechnology companies, is expanding in vaccine distribution and awareness initiatives. Partnerships with hospitals, clinics, and retail pharmacies provide greater accessibility. Growing collaborations between private and public stakeholders continue to transform delivery models, leading to improved vaccination rates worldwide.
- For Instance,In a clinical trial comparing varicella vaccines in 2022, a competitor’s vaccine was shown to be non-inferior to Merck’s VARIVAX. In that study, involving children aged 12–15 months, the seroresponse rate for the VARIVAX group was 98.88% (n=484).
Market Challenges Analysis:
High Costs, Limited Access, and Infrastructure Barriers:
The varicella vaccine market faces challenges linked to high vaccination costs and uneven access in low-income regions. Many healthcare systems struggle with limited budgets, which restricts large-scale immunization efforts. Cold-chain requirements for vaccine storage further increase logistical difficulties in rural and remote areas. It places additional pressure on governments and health agencies that aim to expand coverage. Inconsistent reimbursement policies across countries reduce patient willingness to pay for vaccination. These barriers create gaps in immunization rates and hinder global disease control goals.
Public Awareness Gaps and Vaccine Hesitancy Concerns:
The varicella vaccine market is also affected by limited awareness and ongoing vaccine hesitancy. In several regions, caregivers underestimate the risks of chickenpox, leading to lower demand for vaccination. Misconceptions about vaccine safety continue to spread, despite strong clinical evidence of effectiveness. It weakens public trust and slows adoption across vulnerable populations. Cultural beliefs and misinformation campaigns can discourage immunization efforts in specific markets. Addressing these challenges requires consistent education programs and transparent communication from healthcare providers. Growing reliance on trusted local networks can help counter hesitancy and improve uptake.
Market Opportunities:
Expansion in Emerging Economies and Growing Pediatric Demand
The varicella vaccine market offers strong opportunities in emerging economies with expanding healthcare infrastructure. Governments in Asia-Pacific, Latin America, and Africa are investing in routine immunization programs to address childhood diseases. A large pediatric population creates sustained demand for effective vaccines. It encourages manufacturers to strengthen local partnerships and expand distribution networks. Rising healthcare expenditure and supportive policies further improve accessibility in underserved areas. These conditions position emerging regions as high-growth markets for vaccine providers.
Innovation in Formulations and Strategic Collaborations
The varicella vaccine market is poised to benefit from innovation in combination vaccines and next-generation formulations. Research efforts focus on improving dosage efficiency, stability, and long-term protection. It opens new possibilities for wider use in public health programs. Collaborations between pharmaceutical companies, biotech firms, and government agencies enhance R&D capabilities and global distribution. Expanding partnerships with private healthcare providers also improve awareness and delivery efficiency. These opportunities highlight a favorable environment for sustained industry growth.
Market Segmentation Analysis:
By Product:
The varicella vaccine market is segmented into monovalent and combination vaccines. Monovalent vaccines remain widely used due to their targeted protection and established safety profile. Combination vaccines that include protection against measles, mumps, and rubella are gaining traction. It reduces the number of injections required, improves compliance, and lowers overall healthcare costs. The rising demand for efficient immunization schedules supports stronger adoption of these combination products across global markets.
- For Instance, GSK’s Priorix-Tetra is a real vaccine that combines protection against varicella, measles, mumps, and rubella, streamlining immunization schedules for young children. As a major vaccine manufacturer, GSK distributes its vaccines globally, including within the European market.
By Administration Route:
The market is divided into subcutaneous and intramuscular routes. Subcutaneous administration dominates, supported by its established safety and ease of use in pediatric populations. Intramuscular routes are preferred in specific healthcare settings where faster absorption is required. It provides flexibility for physicians and healthcare workers, ensuring coverage across varied patient groups. The availability of both methods allows health systems to expand vaccination programs without limiting adoption.
- For Instance,Pfizer’s Prevnar 20 vaccine is administered via intramuscular injection. Intramuscular injections are generally absorbed faster than subcutaneous injections.
By End Use:
The market is classified into hospitals, clinics, and others such as community health centers. Hospitals account for the largest share due to structured immunization programs and advanced facilities. Clinics also play a critical role, especially in urban and semi-urban regions with growing vaccine demand. It helps extend access to populations that may not rely on large healthcare institutions. Community centers in developing regions are expected to drive further expansion, supported by government-backed vaccination campaigns.
Segmentations:
By Product:
- Monovalent Vaccines
- Combination Vaccines
By Administration Route:
- Subcutaneous
- Intramuscular
By End Use:
- Hospitals
- Clinics
- Community Health Centers
- Others
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America:
North America accounted for 41% market share in 2024, making it the leading region. The dominance of the region is supported by high vaccination coverage, advanced healthcare infrastructure, and government-backed immunization policies. The varicella vaccine market in North America also benefits from favorable reimbursement frameworks and strong pharmaceutical presence. It continues to grow as healthcare providers integrate vaccines into routine pediatric care. The United States remains the primary contributor, supported by strong awareness campaigns and CDC recommendations. Canada further strengthens the region’s position through national vaccination initiatives.
Europe:
Europe held 28% market share in 2024, ranking as the second-largest region globally. Strong national immunization programs, supportive regulations, and public funding drive growth. The varicella vaccine market in Europe expands further as countries adopt combination vaccines and focus on disease prevention. It gains strength from advanced infrastructure and partnerships with leading manufacturers. Germany, the United Kingdom, and France represent the largest contributors, supported by robust healthcare spending. Rising emphasis on sustainable and high-quality vaccine supply chains enhances adoption across the region.
Asia-Pacific:
Asia-Pacific secured 21% market share in 2024, with rapid growth projected through the forecast period. A large pediatric population and expanding healthcare investments make the region highly attractive. The varicella vaccine market in Asia-Pacific is driven by rising immunization coverage and government-supported campaigns. It continues to benefit from improved distribution networks and increasing awareness levels. China and India dominate regional demand due to large-scale national vaccination drives. Japan and South Korea further strengthen growth through advanced healthcare systems and supportive policy frameworks.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- VBI Vaccines
- MedImmune
- Sanofi
- Novartis
- Kaketsuken
- GlaxoSmithKline
- Pfizer
- Inovio Pharmaceuticals
- AstraZeneca
- Merck and Co
- Emergent BioSolutions
Competitive Analysis:
The varicella vaccine market is highly competitive with global and regional players driving innovation and distribution. Key companies include VBI Vaccines, MedImmune, Sanofi, Novartis, Kaketsuken, and GlaxoSmithKline. These firms focus on strengthening product portfolios, improving vaccine stability, and expanding access through strategic partnerships. It emphasizes efficiency by investing in advanced research and leveraging established healthcare networks. Companies also compete by aligning with government immunization programs and public health organizations to expand coverage. Growth strategies often include mergers, acquisitions, and collaborations aimed at enhancing distribution reach in emerging economies. The competition reflects a balance between innovation, affordability, and compliance with stringent regulatory frameworks.
Recent Developments:
- In February 2024, Brii Biosciences entered agreements to acquire all intellectual property rights from VBI Vaccines related to BRII-179 and to assume manufacturing responsibilities for BRII-179 and PreHevbrio following June 2024.
- In July 2025, Sanofi completed the acquisition of Blueprint Medicines, gaining Ayvakit/Ayvakyt and other pipeline assets.
Report Coverage:
The research report offers an in-depth analysis based on Product, Administration Route, End Use and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The varicella vaccine market will expand with wider integration into national immunization schedules.
- Pharmaceutical companies will invest in advanced formulations to improve long-term protection and stability.
- Public-private partnerships will strengthen vaccine accessibility in underserved and rural regions.
- Healthcare providers will increasingly adopt combination vaccines to enhance efficiency and compliance.
- Rising pediatric populations in Asia-Pacific and Africa will drive higher vaccination demand.
- Digital health tools will support tracking of vaccine coverage and patient compliance.
- Government initiatives will focus on strengthening awareness campaigns to reduce vaccine hesitancy.
- Biotechnology innovations will enable faster production and improved vaccine quality standards.
- Global collaborations will enhance supply chain resilience and ensure steady vaccine distribution.
- The market will benefit from consistent policy support that prioritizes preventive healthcare.

 Market Insights:
Market Insights:

