Market Overview
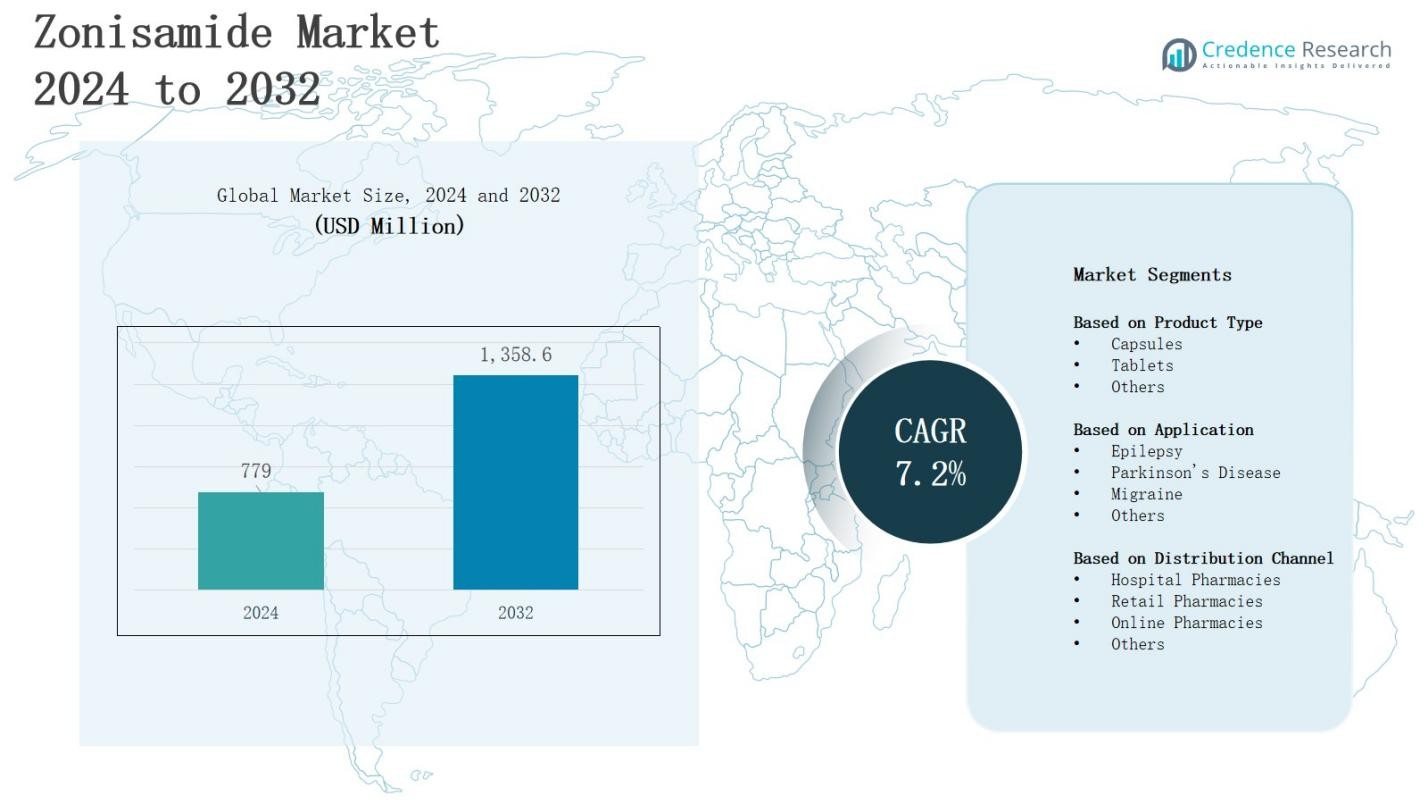
The Zonisamide Market is projected to grow from USD 779 million in 2024 to USD 1,358.6 million by 2032, registering a CAGR of 7.2% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Zonisamide Market Size 2024 |
USD 779 Million |
| Zonisamide Market, CAGR |
7.2% |
| Zonisamide Market Size 2032 |
USD 1,358.6 Million |
The Zonisamide Market is driven by rising prevalence of epilepsy and other neurological disorders, growing awareness of early diagnosis, and increasing adoption of adjunctive therapies to improve seizure control. Expanding applications in off-label uses, coupled with advancements in formulation for enhanced patient compliance, further support demand. Trends include the development of extended-release formulations, strategic collaborations between pharmaceutical companies for distribution and R&D, and a focus on emerging markets with rising healthcare access. Integration of digital health tools for patient monitoring and personalized treatment approaches is also shaping market evolution, creating opportunities for sustained growth.
The zonisamide market spans North America, Europe, Asia-Pacific, and the Rest of the World, with North America holding the largest share, followed by Europe and Asia-Pacific, while the Rest of the World shows steady growth. It is supported by strong demand in developed regions and expanding access in emerging markets. Key players include Eisai Co., Ltd., Sun Pharmaceutical Industries Ltd., Zydus Cadila, Glenmark Pharmaceuticals Ltd., Teva Pharmaceutical Industries Ltd., Mylan N.V., Apotex Inc., Dr. Reddy’s Laboratories Ltd., Lupin Limited, Aurobindo Pharma Ltd., Torrent Pharmaceuticals Ltd., and Hikma Pharmaceuticals PLC.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The zonisamide market is projected to grow from USD 779 million in 2024 to USD 1,358.6 million by 2032, at a CAGR of 7.2% during the forecast period.
- Rising prevalence of epilepsy and other neurological disorders, along with growing adoption of adjunctive therapies, is driving market demand.
- Advancements in extended-release formulations and patient-friendly dosage forms are improving adherence and expanding treatment options.
- Increasing off-label use and combination therapy adoption are broadening clinical applications beyond epilepsy.
- North America holds 38% market share, Europe 27%, Asia-Pacific 24%, and Rest of the World 11%.
- Challenges include adverse side effects impacting compliance and intensified price competition from generics after patent expirations.
- Key players include Eisai Co., Ltd., Sun Pharmaceutical Industries Ltd., Zydus Cadila, Glenmark Pharmaceuticals Ltd., Teva Pharmaceutical Industries Ltd., Mylan N.V., Apotex Inc., Dr. Reddy’s Laboratories Ltd., Lupin Limited, Aurobindo Pharma Ltd., Torrent Pharmaceuticals Ltd., and Hikma Pharmaceuticals PLC.
Market Drivers
Rising Prevalence of Epilepsy and Neurological Disorders
The zonisamide market is experiencing strong growth due to the increasing global incidence of epilepsy and other neurological disorders. It is widely prescribed as an adjunctive therapy to manage partial seizures in adults and children. Growing diagnostic capabilities and heightened public awareness are expanding patient identification rates. Demand is further strengthened by the need for long-term seizure control. Expanding access to healthcare services in emerging markets broadens the treatment base, supporting sustained market expansion.
Advancements in Drug Formulations and Delivery Methods
Technological progress in pharmaceutical manufacturing is driving demand in the zonisamide market. It benefits from the development of extended-release and patient-friendly dosage forms that improve adherence and reduce dosing frequency. Formulation improvements aim to minimize side effects while maintaining therapeutic efficacy. These innovations support treatment personalization, enabling healthcare providers to meet diverse patient needs. Regulatory approvals for improved formulations strengthen competitive positioning and create new revenue opportunities for market players.
- For instance, Viatris offers zonisamide 100 mg hard capsules approved for monotherapy and adjunctive therapy in epilepsy treatment, focusing on controlled dosing regimens for optimal therapeutic effect and patient safety.
Increasing Adoption in Off-Label and Adjunctive Therapies
The zonisamide market is expanding due to its growing use in off-label applications and as an adjunctive therapy for treatment-resistant cases. Clinicians often combine it with other antiepileptic drugs to enhance seizure control, especially in complex patient profiles. Its pharmacological profile allows flexibility in combination therapy without significant drug interactions. This versatility increases its adoption in diverse clinical scenarios. Rising clinical evidence supports its broader therapeutic potential, driving its integration into advanced treatment protocols.
- For instance, in Japan, zonisamide has been used as an adjunct to levodopa since 2009 to improve motor symptoms in Parkinson’s disease patients.
Expansion in Emerging Markets and Healthcare Infrastructure Growth
The zonisamide market benefits from rapid improvements in healthcare infrastructure and access in developing regions. Growing investment in neurology-focused healthcare facilities expands diagnostic and treatment capabilities. It gains traction as more patients receive timely and accurate diagnoses. Favorable reimbursement policies and government healthcare initiatives further support uptake. Rising disposable incomes and better availability of branded and generic versions make it more accessible, strengthening its presence in high-growth regional markets.
Market Trends
Development of Extended-Release and Patient-Centric Formulations
The zonisamide market is witnessing a strong shift toward extended-release and patient-friendly dosage forms to enhance compliance and treatment outcomes. It is benefiting from innovations that reduce dosing frequency while maintaining consistent therapeutic levels. Pharmaceutical companies are focusing on minimizing side effects through targeted delivery systems. These advancements address patient adherence challenges in long-term therapy. Continuous research in drug release mechanisms is creating competitive differentiation, supporting higher adoption in both developed and emerging healthcare markets.
Integration of Digital Health Solutions for Monitoring and Management
The zonisamide market is increasingly influenced by the adoption of digital health tools that enable real-time monitoring of patient adherence and seizure patterns. It is supported by telemedicine platforms and wearable devices that help clinicians adjust treatment plans more effectively. Data-driven insights from connected health systems are improving clinical decision-making. This trend is fostering better patient engagement and outcomes. Partnerships between pharmaceutical firms and digital health companies are accelerating integrated care models in neurology.
- For instance, Eisai Co., Ltd., the original rights holder of Zonegran, continues to distribute zonisamide in select markets while focusing on strategic resource allocation to digital health and patient-centric care models, supporting improved clinical decision-making through integrated data platforms.
Strategic Collaborations and Licensing Agreements for Market Expansion
The zonisamide market is seeing an upsurge in strategic collaborations, licensing deals, and co-marketing agreements aimed at expanding geographical reach and product portfolios. It is benefiting from alliances that enable faster entry into high-growth regions. Such partnerships reduce regulatory and distribution barriers, allowing companies to optimize commercialization strategies. Joint research initiatives are accelerating clinical studies for new therapeutic uses. This approach enhances market penetration while reducing the time and cost of development.
Rising Focus on Generic Production and Cost-Effective Access
The zonisamide market is shaped by the growing presence of generic manufacturers offering cost-effective alternatives to branded drugs. It is gaining momentum in price-sensitive regions where affordability drives prescription volumes. Competitive pricing is expanding patient access without compromising quality standards. Manufacturers are investing in advanced production technologies to meet stringent regulatory requirements. This trend is fostering broader adoption, particularly in emerging economies, while intensifying competition among established and new market entrants.
- For instance, Abbott Healthcare Pvt. Ltd. and Sun Pharmaceuticals Industries Ltd. are among key manufacturers producing affordable zonisamide capsules, thereby expanding patient access while maintaining quality standards.
Market Challenges Analysis
Adverse Effects and Safety Concerns Impacting Patient Compliance
The zonisamide market faces challenges related to its potential side effects, which can range from mild symptoms such as dizziness and loss of appetite to serious concerns including metabolic acidosis, kidney stones, and cognitive impairment. It is often necessary for healthcare providers to monitor patients closely, which can deter widespread adoption. Safety concerns are especially significant in pediatric and elderly populations, requiring careful dosage adjustments. Negative patient experiences may lead to treatment discontinuation. These risks contribute to hesitancy among clinicians when considering zonisamide for long-term therapy.
Patent Expiry and Intensifying Generic Competition
The zonisamide market is under pressure from patent expirations that have opened the door to generic competition. It is experiencing price erosion in multiple regions, reducing profitability for branded manufacturers. Generic versions attract cost-conscious healthcare systems and patients, particularly in emerging economies. While this expands accessibility, it limits revenue growth for innovators and reduces incentives for further R&D investments. Competitive pricing strategies from generics also challenge market leaders to differentiate through innovation, brand loyalty, and value-added formulations.
Market Opportunities
Expansion into Emerging Markets with Growing Healthcare Access
The zonisamide market has significant growth potential in emerging economies where improving healthcare infrastructure and increasing awareness of epilepsy treatment are creating new demand. It is benefiting from government initiatives to expand neurological care services and subsidize essential medicines. Rising disposable incomes are enabling more patients to afford advanced therapies. Wider availability of both branded and generic options supports broader adoption. Targeted marketing and physician education programs can further accelerate penetration in these high-growth regions.
Advancements in Formulations and Broader Therapeutic Applications
The zonisamide market offers opportunities through the development of advanced formulations that enhance patient compliance and minimize adverse effects. It is well-positioned for research into extended-release versions and novel delivery systems that address current limitations. Ongoing clinical studies exploring potential applications in other neurological or psychiatric conditions could expand its therapeutic footprint. Collaborations between pharmaceutical companies and research institutions can fast-track these innovations. Successful diversification of indications will create long-term growth avenues and strengthen market resilience.
Market Segmentation Analysis:
By Product Type
The zonisamide market is segmented into capsules, tablets, and others. Capsules hold a substantial share due to ease of swallowing, precise dosing, and higher patient acceptance in long-term therapies. Tablets remain a widely used format for cost efficiency and stability in storage. The “others” category includes liquid suspensions and compounded formulations for patients with swallowing difficulties. It benefits from ongoing innovations aimed at improving bioavailability and patient compliance across all product types.
- For instance, Zonegran capsules are available in 25 mg, 50 mg, and 100 mg doses, formulated to ensure accurate dosage and patient compliance in epilepsy treatment. Tablets remain a widely used format for cost efficiency and stability in storage.
By Application
The zonisamide market is categorized into epilepsy, Parkinson’s disease, migraine, and others. Epilepsy dominates the segment owing to the drug’s established role as an adjunctive therapy for partial seizures. Parkinson’s disease applications are growing due to its potential benefits in managing motor symptoms. Migraine treatment is emerging as a niche application supported by exploratory clinical research. The “others” segment includes mood disorders and off-label uses, creating opportunities for therapeutic diversification.
- For instance, zonisamide has been demonstrated to significantly reduce headache days per month in pediatric migraine patients, with a median decrease from 18 to 6 days after starting treatment, supporting its emerging use in migraine prophylaxis.
By Distribution Channel
The zonisamide market is divided into hospital pharmacies, retail pharmacies, online pharmacies, and others. Hospital pharmacies lead due to high prescription volumes from neurology specialists and in-patient care settings. Retail pharmacies provide wide accessibility and remain a preferred choice for recurring prescriptions. Online pharmacies are expanding rapidly, driven by convenience and competitive pricing. The “others” segment covers specialty drug distributors, serving institutions and clinics with targeted supply needs.
Segments:
Based on Product Type
Based on Application
- Epilepsy
- Parkinson’s Disease
- Migraine
- Others
Based on Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Based on the Geography:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis
North America
North America holds 38% of the zonisamide market, driven by advanced healthcare infrastructure, high diagnosis rates of epilepsy, and strong adoption of adjunctive therapies. It benefits from the presence of leading pharmaceutical companies and favorable reimbursement policies. Clinical research activities are well-supported by government and private funding, encouraging new formulation development. Patient awareness programs and access to specialized neurology care strengthen uptake. Regulatory compliance remains robust, ensuring high-quality manufacturing standards. Expansion in telehealth services is further improving treatment accessibility across urban and rural areas.
Europe
Europe accounts for 27% of the zonisamide market, supported by strong demand in countries with established neurology treatment frameworks such as Germany, France, and the UK. It is influenced by government healthcare programs that cover antiepileptic drugs under public insurance schemes. Widespread physician familiarity with zonisamide ensures steady prescription volumes. Research collaborations between academic institutions and pharmaceutical firms are fostering innovation. The market also benefits from the region’s strong generic manufacturing base, enhancing affordability. Aging populations with higher neurological disorder prevalence are expanding the patient pool.
Asia-Pacific
Asia-Pacific represents 24% of the zonisamide market, fueled by rising healthcare access, growing awareness of epilepsy management, and increasing healthcare investments in countries like China, Japan, and India. It is supported by government initiatives to improve neurological care services in both urban and rural areas. Expanding distribution networks ensure wider drug availability. Local manufacturing capabilities reduce costs and improve affordability. Multinational companies are entering partnerships with regional players to strengthen market penetration. Rising middle-class income levels further support demand growth.
Rest of the World
The Rest of the World holds 11% of the zonisamide market, encompassing Latin America, the Middle East, and Africa. It is influenced by gradual improvements in healthcare infrastructure and increasing access to neurological care. Government and non-government health programs are working to improve epilepsy diagnosis and treatment availability. Limited specialist availability in some areas remains a challenge, but telemedicine is helping bridge gaps. Generic versions are expanding access in cost-sensitive markets. Pharmaceutical companies are targeting these regions through strategic supply agreements and awareness initiatives.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Glenmark Pharmaceuticals Ltd.
- Zydus Cadila
- Lupin Limited
- Torrent Pharmaceuticals Ltd.
- Hikma Pharmaceuticals PLC
- Apotex Inc.
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Reddy’s Laboratories Ltd.
- Sun Pharmaceutical Industries Ltd.
- Aurobindo Pharma Ltd.
- Eisai Co., Ltd.
Competitive Analysis
The zonisamide market is characterized by strong competition among global and regional pharmaceutical companies focusing on both branded and generic formulations. It is driven by strategic initiatives such as product innovation, cost optimization, and geographic expansion. Key players include Eisai Co., Ltd., Sun Pharmaceutical Industries Ltd., Zydus Cadila, Glenmark Pharmaceuticals Ltd., Teva Pharmaceutical Industries Ltd., Mylan N.V., Apotex Inc., Dr. Reddy’s Laboratories Ltd., Lupin Limited, Aurobindo Pharma Ltd., Torrent Pharmaceuticals Ltd., and Hikma Pharmaceuticals PLC. These companies compete on product quality, pricing strategies, and distribution reach to strengthen market share. Innovation in extended-release formulations, partnerships for distribution, and expansion in emerging economies remain critical strategies. Regulatory compliance, supply chain efficiency, and clinical trial advancements further shape competitive positioning, while generic manufacturers intensify price competition and broaden accessibility.
Recent Developments
- On August 8, 2025, Zydus Lifesciences received final approval from the US FDA for Prucalopride Tablets, 1 mg and 2 mg. (Note: While not zonisamide, it signals recent drug approvals by the company in neurology/related therapy areas.)
- In March 2025, Azurity Pharmaceuticals completed the acquisition of Covis Group S.à r.l., strengthening its pharmaceutical portfolio and operational capabilities. The company remains a notable player in the zonisamide market, having previously introduced Zonisade™, the first FDA-approved oral suspension of zonisamide.
- On December 16, 2024, Glenmark Pharmaceuticals Inc., USA, launched Lacosamide Oral Solution, 10 mg/mL in the U.S. market, which is an antiepileptic drug, strengthening their portfolio in neurology therapeutics.
- In July 2022, Azurity Pharmaceuticals received FDA approval for Zonisade™, the first oral suspension of zonisamide, offering a convenient alternative for patients unable to swallow capsules.
Market Concentration & Characteristics
The zonisamide market exhibits a moderately high level of concentration, with a mix of global pharmaceutical leaders and strong regional manufacturers competing across branded and generic segments. It is characterized by intense price competition, especially in markets with established generic penetration following patent expirations. Leading companies focus on formulation innovation, geographic expansion, and strategic partnerships to sustain market share. Regulatory compliance, manufacturing quality, and distribution efficiency remain critical success factors. The market benefits from steady demand driven by epilepsy prevalence and expanding therapeutic applications. Key players actively invest in R&D for extended-release versions and improved delivery systems to differentiate from low-cost generics. Growing access to healthcare in emerging economies and wider online pharmacy penetration are enhancing product availability. Market dynamics are further shaped by competitive pricing strategies, targeted marketing, and efforts to build strong brand recognition among healthcare providers and patients.
Report Coverage
The research report offers an in-depth analysis based on Product Type, Application, Distribution Channel and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Demand will rise with increasing epilepsy diagnosis rates and improved access to neurological care.
- Extended-release and patient-friendly formulations will gain wider adoption.
- Off-label applications will expand therapeutic use beyond epilepsy.
- Digital health integration will enhance patient monitoring and treatment adjustments.
- Generic competition will intensify in cost-sensitive markets.
- Strategic collaborations will support faster market penetration in emerging economies.
- Regulatory approvals for new formulations will strengthen competitive positioning.
- Online pharmacy growth will improve global distribution and accessibility.
- R&D investments will focus on minimizing side effects and improving safety profiles.
- Growing middle-class populations in developing regions will boost prescription volumes.




