Rising Incidence of Hospital-Acquired Infections and Adoption of Automated Reprocessing Systems Fuel Market Growth
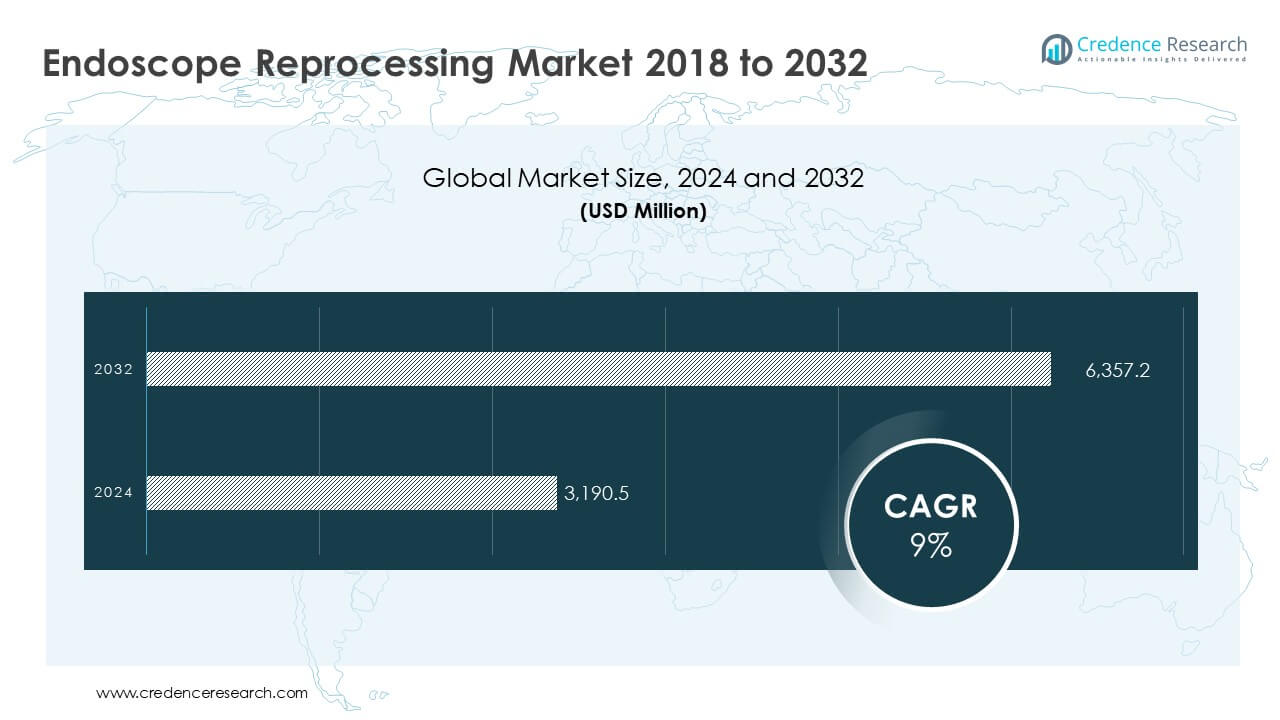
According to the latest report published by Credence Research, “Endoscope Reprocessing Market – Growth, Share, Opportunities & Competitive Analysis, 2024 – 2032,” the global Endoscope Reprocessing Market was valued at USD 3,190.5 million in 2024 and is projected to reach USD 6,357.2 million by 2032, expanding at a CAGR of 9.0% over the forecast period.
The market’s expansion is underpinned by growing procedural volumes across gastroenterology, pulmonology, urology, and other specialties, combined with strict infection prevention mandates. Advancements in sterilization and traceability technologies, paired with regulatory compliance pressures, are driving demand for more efficient, automated endoscope reprocessing systems across healthcare facilities globally.
Browse market data Figures spread through 220 + Pages and an in-depth TOC on “Endoscope Reprocessing Market”
Rising Incidence of Hospital-Acquired Infections (HAIs) Drives Demand for Advanced Reprocessing Protocols
A key growth driver for the global Endoscope Reprocessing Market is the increasing global concern surrounding hospital-acquired infections (HAIs). Flexible endoscopes, widely used in diagnostic and therapeutic procedures, are highly prone to contamination due to their intricate design. Their complex lumens and channels are difficult to clean using conventional sterilization methods, increasing the risk of cross-contamination and infection transmission between patients.
To mitigate this risk, healthcare providers are investing in advanced reprocessing technologies such as Automated Endoscope Reprocessors (AERs) and high-level disinfectants. These systems are capable of ensuring consistent, repeatable disinfection cycles while adhering to strict regulatory standards. The integration of chemical sterilants, combined with validated cleaning protocols, enhances infection control effectiveness.
The increasing prevalence of chronic diseases has led to a rise in endoscopic interventions, further necessitating robust sterilization measures. With growing regulatory scrutiny and patient safety initiatives, demand continues to rise for traceable, automated reprocessing systems that minimize human error and deliver consistent cleaning outcomes. These systems help hospitals meet compliance requirements, reduce liability, and prevent infection outbreaks.
Healthcare institutions across developed and emerging economies are increasingly prioritizing infection control infrastructure, aligning their procurement decisions with global standards such as ISO 15883-4. This shift is resulting in widespread adoption of automated and semi-automated reprocessing systems that combine cleaning, disinfection, drying, and traceability into a unified workflow.
Integration of Automation and Digital Traceability Enhances Operational Efficiency
A prominent trend shaping the Endoscope Reprocessing Market is the widespread integration of automation and digital tracking technologies. Automation is redefining endoscope reprocessing workflows by eliminating manual handling steps, thereby reducing variability and increasing compliance with reprocessing protocols.
Modern Automated Endoscope Reprocessors are equipped with integrated barcode scanning, RFID tagging, and data logging capabilities. These features allow healthcare facilities to track every endoscope reprocessing cycle, ensuring that each instrument is fully traceable to a specific procedure and patient case. This level of traceability is critical for quality assurance and regulatory audits, particularly in light of increasing documentation requirements from global health authorities.
Digital integration is also enabling remote monitoring and predictive maintenance of reprocessing units via cloud-based platforms. Such connectivity ensures that any deviations or malfunctions in reprocessing cycles can be quickly identified and resolved. Additionally, these systems can be integrated with hospital information systems (HIS), central sterile services departments (CSSDs), and electronic medical records (EMRs), creating a seamless data ecosystem.
For example, Getinge’s ED-Flow AER processes up to 16 flexible endoscopes per hour and utilizes RFID-based tracking to achieve complete traceability per ISO 15883-4 standards. This trend reflects the broader movement toward data-driven, outcome-focused healthcare delivery, where endoscope reprocessing is positioned as a critical component of patient safety.
The convergence of automation, digital traceability, and regulatory compliance is streamlining reprocessing workflows and enabling facilities to scale operations without compromising on safety or efficiency. This trend is expected to further intensify as healthcare systems worldwide seek to modernize their infection prevention protocols in alignment with global accreditation standards.
Limited Awareness and Infrastructural Constraints in Emerging Economies
Despite growing global demand, the Endoscope Reprocessing Market faces several challenges, particularly in low-resource settings. A significant constraint is the limited awareness regarding the importance of standardized reprocessing practices among healthcare professionals in developing regions. This knowledge gap results in continued reliance on manual cleaning methods that are inconsistent and difficult to validate.
In addition, many small- and mid-sized healthcare facilities in emerging economies lack access to the infrastructure required for high-level reprocessing. Capital-intensive systems such as automated endoscope reprocessors and drying cabinets often remain inaccessible due to budget limitations. Inadequate training of personnel, substandard water quality, and insufficient physical space for dedicated sterile processing units further hinder adoption.
Moreover, compliance with global standards such as ISO and CDC guidelines requires investments in monitoring, validation, and record-keeping systems, which many healthcare providers are unable to afford. This creates disparities in patient safety outcomes between high-income and low-income countries, limiting market penetration in key regions.
Addressing this challenge requires collaborative efforts from manufacturers, governments, and international health bodies. Educational campaigns, equipment subsidies, and scalable, low-cost automation solutions are essential to improving adoption rates in under-resourced regions. Strategic partnerships and regulatory harmonization can further support long-term market development by increasing accessibility and improving baseline reprocessing standards globally.
Key Players
- Olympus
- Ecolab
- ASP
- Getinge
- Belimed
- Shinva Medical Instrument
- Metrex
- ARC Group of Companies
- CONMED Corporation
- Creo Medical
Market Segmentation
By Product
- High-Level Disinfectants & Test Strips
- Detergents & Enzymatic Wipes
- Automated Endoscope Reprocessors (AER)
- Manual Cleaning Stations
- Endoscope Drying, Storage & Transport Cabinets
By Endoscope Modality
- Flexible Endoscopes
- Rigid Endoscopes
- Robot-Assisted Endoscopes
By Application
- Gastro-intestinal Endoscopy
- Pulmonology & Bronchoscopy
- Urology & Gynaecology
- ENT & Laparoscopy
By End-User
- Hospitals
- Ambulatory Surgery Centres (ASC)
The global Endoscope Reprocessing Market spans North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa, with regional dynamics influenced by healthcare infrastructure, regulatory environments, and procedural volumes.
North America dominates the market, driven by advanced healthcare facilities, stringent infection control standards, and widespread use of automated systems. The region benefits from strong adoption of traceability solutions and proactive compliance with CDC and FDA guidelines.
Europe maintains a robust position through standardized clinical protocols, rigorous quality audits, and a strong emphasis on patient safety. The region’s regulatory coherence supports uniform adoption across both public and private healthcare settings.
About Us:
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 2000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives.
Contact Us
Credence Research Inc.
North America – +1 304 308 1216
Australia – +61 4192 46279
Asia Pacific – +81 5050 50 9250
+64 22 017 0275
India – +91 6232 49 3207
sales@credenceresearch.com
www.credenceresearch.com