Market Overview:
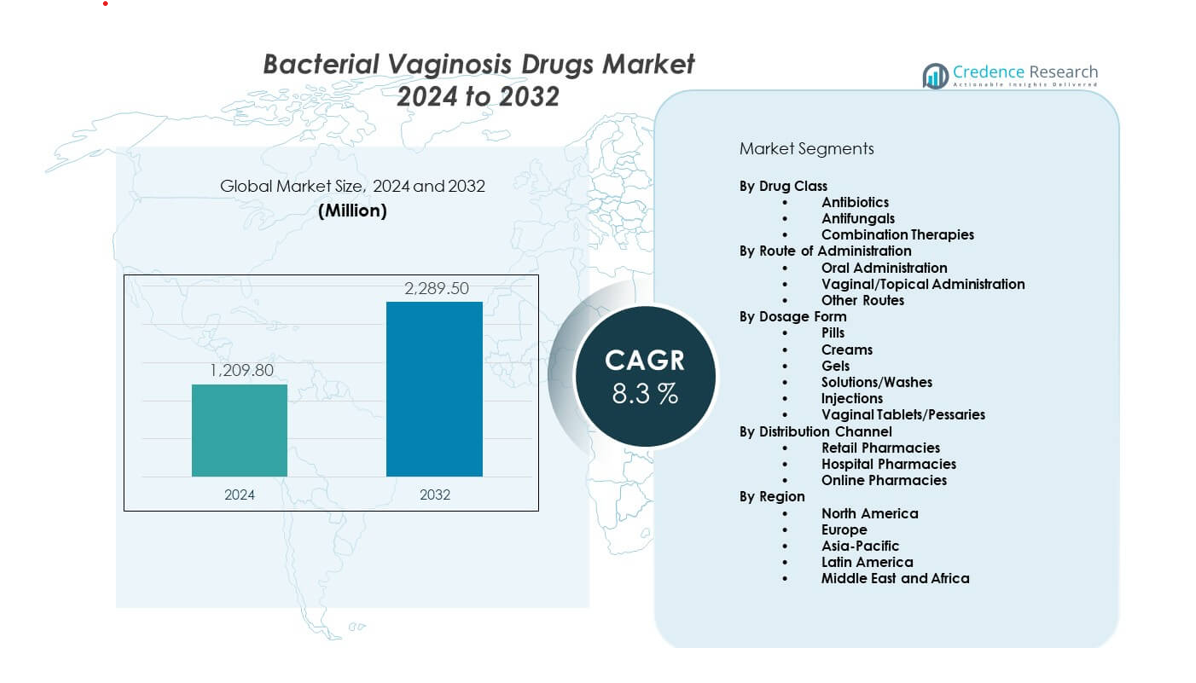
The Bacterial Vaginosis Drugs Market is projected to grow from USD 1,209.8 million in 2024 to an estimated USD 2,289.5 million by 2032, with a CAGR of 8.3% from 2024 to 2032.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Bacterial Vaginosis Drugs Market Size 2024 |
USD 1,209.8 million |
| Bacterial Vaginosis Drugs Market, CAGR |
8.3% |
| Bacterial Vaginosis Drugs Market Size 2032 |
USD 2,289.5 million |
Growing treatment demand comes from higher infection recurrence and improved screening in primary centers. Doctors focus on therapies that shorten recovery time and reduce relapse risk. Drug makers invest in broader antimicrobial profiles that address resistant strains. Hospitals and clinics adopt updated care guidelines, which help raise prescription rates. Women seek safer formulations that offer better comfort and fewer side effects. Research groups advance new delivery formats that improve dosage control and patient confidence.
North America leads due to strong access to branded therapies, better patient awareness, and wider adoption of clinical guidelines. Europe follows with steady demand supported by active women’s health programs and high diagnostic coverage. Asia Pacific grows fast driven by expanding gynecology infrastructure and higher infection prevalence in developing countries. China and India emerge as key contributors due to their large patient base and rising health investment. Latin America records steady uptake as more women seek formal treatment in urban regions. The Middle East and Africa show gradual adoption supported by improving healthcare access.
Market Insights:
- The Bacterial Vaginosis Drugs Market is projected to grow from USD 1,209.8 million in 2024 to USD 2,289.5 million by 2032, reflecting a CAGR of 8.3% driven by rising diagnosis rates and broader treatment access.
- North America leads with 40.5% share, followed by Europe at 30%, and Asia-Pacific at 24%, supported by strong healthcare systems, structured screening programs, and high treatment awareness.
- Asia-Pacific is the fastest-growing region with 24% share, driven by expanding urban populations, improved women’s health access, and rising awareness of reproductive care.
- Antibiotics dominate the drug class segment with 42% share, reflecting strong clinical preference for metronidazole and clindamycin.
- Oral administration leads the route-of-administration segment with 59% share, supported by ease of dosing and widespread adoption across primary care.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Strong Rise In Diagnosis Rates And Awareness Across Women’s Health Programs
Growing screening programs lift early detection across clinics and primary care units. Women seek faster relief options due to rising awareness of recurrent infections. Doctors promote updated treatment guidelines that push higher drug adoption. Hospitals widen diagnostic support for underserved groups that face frequent episodes. The Bacterial Vaginosis Drugs Market gains steady traction through structured awareness drives. Pharma brands support these programs with education tools for communities. Online health platforms ease access to information that guides timely treatment. Early reporting of symptoms improves therapy uptake and strengthens market growth.
- For instance, Hologic’s diagnostic platforms, including the Panther System used in thousands of laboratories globally, process tens of millionsof women’s health tests annually, significantly improving the detection of various infections.
Shift Toward Effective Therapies With Lower Recurrence Probability
Women prefer treatment plans that reduce discomfort and minimize relapse frequency. Drug makers invest in broader antimicrobial profiles that help manage resistant strains. Clinics adopt therapies that provide stable recovery and predictable outcomes. Providers recommend options with fewer side effects to raise patient compliance. The market benefits from drug lines that support flexible dosages and quick relief. It gains stronger trust from patients seeking better long-term control. Hospitals prioritize improved protocols to reduce repeated infection cycles. Doctors emphasize tailored care to match individual health needs.
- For instance, Lupin’s Solosec® (secnidazole) achieved a 53.3% clinical cure rate at day 21–30 in one pivotal trial. This figure demonstrated statistical superiority over a placebo, and the drug’s single-dose regimen is considered an effective, convenient therapeutic alternative to multi-day treatments, which may improve patient adherence.
Growing Demand For Non-Invasive Drug Delivery Formats And Enhanced Comfort
Topical solutions attract steady interest due to ease of use and reduced irritation risk. Many women prefer formats that support private use at home. Pharma companies develop advanced gels and creams with refined stability profiles. Clinics adopt delivery systems that optimize local absorption for better comfort. The Bacterial Vaginosis Drugs Market strengthens through patient-centric innovation in formulations. Research labs enhance bioavailability features that help raise therapeutic value. Digital consultations help doctors prescribe personalized formats that improve adherence. Innovation lifts overall acceptance of modern treatment lines.
Increase In Reproductive Health Investments Across Public And Private Systems
Governments expand women’s health funding to reduce untreated bacterial infections. Hospitals upgrade lab infrastructure to improve accuracy levels during diagnosis. Drug developers receive support for trials that test next-generation antimicrobial classes. Care networks prioritize vaginal health due to high recurrence in key age groups. The market benefits from wide interest among policymakers to reduce infection burden. It expands through coordinated programs that bring screening to rural clusters. Insurance coverage for outpatient care supports access for varied income groups. Providers emphasize structured follow-ups that improve long-term outcomes.
Market Trends:
Rising Focus On Microbiome-Based Research And Balanced Vaginal Ecology Models
Drug developers explore targeted approaches that stabilize beneficial bacteria. Research teams study microbiome shifts that influence therapy performance. Companies track microbial compositions to design next-level treatment concepts. Clinics review patient microbiome data to guide advanced care plans. The Bacterial Vaginosis Drugs Market moves toward solutions that support healthy flora restoration. Labs collaborate with universities to refine organism isolation methods. New insights help optimize drug pathways for persistent cases. Microbiome innovation shapes future product pipelines in this category.
- For instance, Osel’s live biotherapeutic strain Lactobacillus crispatus CTV-05 demonstrated a 30–50% reduction in recurrence risk in NIH-supported clinical studies aimed at restoring vaginal flora.
Growth Of Virtual Gynecology Consultations And Remote Prescription Models
Telehealth platforms lift access to treatment guidance for women in remote areas. Virtual visits reduce hesitation for sensitive reproductive health concerns. Doctors evaluate symptoms faster through structured digital triage formats. E-pharmacy networks deliver prescribed drugs with strong privacy measures. Market adoption rises where clinical access is limited by geography. It gains traction among younger populations that rely on mobile health tools. Remote care lowers delays that worsen infection severity. Digital ecosystems strengthen continuity of care across follow-up cycles.
- For instance, Teladoc Health reported approximately 4 million total virtual visitsacross all service categories in 2023, contributing to their full year revenue of over $2.6 billion. While women are significant users of virtual health services and the category is growing, the specific number of women’s health-focused visits was not disclosed in public financial reports.
Expansion Of OTC Formulations With Stronger Compliance And Wider Retail Reach
OTC therapy choices increase for women seeking quick relief options. Retail chains highlight safer vaginal health solutions with clear usage guidance. Manufacturers roll out improved packaging formats that enhance hygiene levels. Pharmacies promote OTC products that support mild-case management. The Bacterial Vaginosis Drugs Market benefits from expanded shelf presence across continents. Low-barrier access attracts new users that avoid clinical visits. Direct-to-consumer education campaigns support OTC awareness. Growing comfort with self-care boosts category growth.
Integration Of Digital Symptom Tracking Tools Into Treatment Journeys
Apps help women track cycles and infection patterns for better clinical insight. Data logs guide doctors toward precise therapy choices and dosage control. Digital tools highlight triggers that influence recurrence risk. Many platforms connect users to certified gynecology experts. Market traction grows as analytics support accurate decision-making. It creates better monitoring for women needing recurrent therapy management. Wearable integrations help identify shifts that warrant early evaluation. Personalized alerts improve patient engagement throughout treatment.
Market Challenges Analysis:
High Recurrence Rates And Limited Long-Term Treatment Success
Recurring infections weaken confidence among women who seek lasting relief. Doctors struggle to maintain sustained results due to complex microbial shifts. Resistant strains reduce the effectiveness of widely used drug classes. Patients face repeated cycles that disrupt adherence and reduce satisfaction. The Bacterial Vaginosis Drugs Market faces pressure to develop stronger and longer-acting therapies. Uneven clinical outcomes challenge providers that manage chronic cases. Many regions lack advanced screening tools that detect underlying risk drivers. Long-term management remains difficult where follow-up care is inconsistent.
Slow Innovation Cycles And Regulatory Barriers Affecting Product Launches
Drug discovery requires long trials that delay access to novel formulations. Regulatory pathways demand extensive evidence that extends approval timelines. Small firms struggle to fund advanced antimicrobial research. It affects the market’s pace of innovation for next-generation solutions. Clinical variability across populations makes standardization difficult for trials. Manufacturers adapt slowly due to cost constraints and limited R&D resources. Health systems hesitate to adopt new drugs without clear comparative data. These hurdles restrict broader advancement across global markets.
Market Opportunities:
Rising Demand For Advanced Formulations Supporting Comfort And Fast Relief
Women seek therapies that shorten treatment windows and provide targeted comfort. Drug makers innovate around gels, extended-release formats, and improved topical agents. Hospitals adopt upgraded care pathways that promote modern formulations. The Bacterial Vaginosis Drugs Market can expand by focusing on ease of use. Digital tools help guide patient selection for advanced products. Clinics embrace personalized protocols for women with recurrent cases. Global awareness programs highlight the importance of timely care. Innovation opens space for premium therapeutic categories.
Expanding Access Through Telehealth, E-Pharmacy, And Rural Outreach Networks
Remote consultation models support women who lack physical access to clinics. E-pharmacy distribution ensures privacy and consistent drug availability. Outreach programs raise awareness in underserved regions with high infection rates. Providers integrate digital follow-ups to improve adherence. Market expansion gains momentum through cross-sector partnerships. Community health teams deliver education that guides proper usage. It strengthens treatment outcomes across varied socioeconomic groups. Wider connectivity supports stronger adoption across global regions.
Market Segmentation Analysis:
By Drug Class
The Bacterial Vaginosis Drugs Market shows strong dominance of antibiotics, with metronidazole and clindamycin leading due to clinical effectiveness. Antifungals hold limited use for specific conditions requiring targeted action. Combination therapies gain interest for recurrent cases where single-agent treatments provide weaker results. Drug developers expand portfolios to meet varied symptom profiles. Clinics prefer agents with predictable outcomes and strong safety records. Hospitals use structured guidelines to streamline drug selection. Recurrent infection trends push demand for broader therapeutic choices. This segment continues to evolve with new formulations under evaluation.
- For instance, metronidazole, a medication sold under brand names like Flagyl®and others, remains one of the most widely prescribed antibiotics for BV, supported by over 40 years of validated clinical data according to FDA information. While various pharmaceutical companies, including Abbott and Sanofi, manufacture it globally, the U.S. rights to the brand name Flagyl® were acquired by G.D. Searle LLC (a company later merged into Pfizer) in 2003, and Pfizer is listed as a current distributor of the branded product in the US.
By Route of Administration
Oral administration remains widely preferred for convenience and systemic reach. It supports high adoption in primary care settings. Vaginal and topical administration offers targeted relief for localized symptoms. Many women choose topical formats to reduce systemic exposure. Other routes such as parenteral delivery remain less common but serve specialized needs. It benefits from innovation that enhances absorption and user comfort. Clinics recommend formats based on severity and patient preference. Routing flexibility strengthens treatment accessibility across varied patient groups.
- For instance, Starpharma’s VivaGel® BV achieved symptom relief improvements supported by repeat-use clinical studies involving more than 400 participants, demonstrating strong performance as a topical option.
By Dosage Form
Pills maintain leadership due to ease of dosing and strong availability. Creams and gels support localized therapy with controlled application. Solutions and washes serve patients requiring pH restoration. Injections remain limited to specific clinical scenarios. Vaginal tablets and pessaries provide sustained local delivery for improved adherence. It expands with advanced formulation research. Pharmacies stock multiple dosage forms to support tailored care. Providers match formulations to symptom patterns and treatment goals.
By Distribution Channel
Retail pharmacies dominate due to wide consumer access and strong OTC uptake. Hospital pharmacies support prescription-based demand for recurrent and acute cases. Online pharmacies rise steadily with discreet delivery and expanded reach. It benefits from higher digital adoption among younger women. Care networks use online channels to support follow-up access. Distribution diversity supports strong patient convenience. Brands optimize packaging for each channel. Market growth aligns with expanding healthcare retail infrastructure.
Segmentation:
By Drug Class
- Antibiotics
- Antifungals
- Combination Therapies
By Route of Administration
- Oral Administration
- Vaginal/Topical Administration
- Other Routes
By Dosage Form
- Pills
- Creams
- Gels
- Solutions/Washes
- Injections
- Vaginal Tablets/Pessaries
By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America and Europe
The Bacterial Vaginosis Drugs Market holds its strongest presence in North America with a market share of 40.5%, supported by advanced healthcare networks and high awareness of women’s health. Clinics adopt structured guidelines that raise prescription rates across primary and specialty care. Strong diagnostic penetration improves early detection, which supports stable demand across drug classes. Europe follows with a significant share of 30%, driven by robust reimbursement systems and active screening programs. Providers in the region emphasize standardized care practices that reduce treatment gaps. Governments support awareness campaigns that strengthen early reporting of symptoms. It sustains reliable growth due to wide acceptance of oral and topical therapies.
Asia-Pacific
Asia-Pacific stands as the fastest-growing region and an expanding share of 24%. Growth comes from rapid urbanization and rising access to women’s health services. Clinics in China and India experience increasing patient flow due to broader awareness of reproductive health. Hospitals upgrade diagnostic infrastructure, which improves treatment accuracy across large populations. The region benefits from stronger pharmacy networks that lift access to oral and topical formulations. Local drug makers expand low-cost options to support underserved groups. It demonstrates high potential due to large reproductive-age demographics.
Latin America, Middle East, and Africa
Latin America holds an emerging share of 8%, supported by rising urbanization and better access to retail pharmacies. Awareness programs improve understanding of BV symptoms across major cities. Clinics adopt structured treatment protocols that strengthen drug demand across segments. The Middle East and Africa account for 6% market share with gradual improvement in healthcare delivery. Investments in women’s health infrastructure help expand screening and treatment accuracy. Rural outreach programs raise adoption in regions with limited clinical access. It progresses steadily due to growing acceptance of modern therapies and wider pharmacy penetration.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Sanofi Aventis, Inc.
- Bayer AG
- Pfizer Inc.
- Novartis AG
- Starpharma Holdings Limited
- Sun Pharmaceutical Industries Ltd.
- Mylan N.V.
- Perrigo Company Plc
- Abbott Laboratories, Inc.
- Viatris Inc.
- Glenmark Pharmaceuticals Ltd.
- Lupin Ltd.
- Teva Pharmaceutical Industries
- Symbiomix Therapeutics
Competitive Analysis:
The Bacterial Vaginosis Drugs Market features strong competition among global and regional pharmaceutical companies focused on antibiotics, topical therapies, and emerging formulations. Leading brands strengthen their presence through broad distribution networks and frequent product updates. It gains momentum from companies investing in improved delivery systems and differentiated formulations that appeal to patient comfort needs. Generic manufacturers expand access by offering cost-effective alternatives in high-demand regions. Innovation from biotech firms introduces advanced topical and combination therapies for recurrent cases. Partnerships between research labs and drug makers support trials for next-generation solutions. Market competition intensifies as firms target improved recurrence control. Strong retail and online pharmacy presence supports higher visibility across all drug classes.
Recent Developments:
- In January 2024, Starpharma announced significant commercial progress for VivaGel BV, its novel non-antibiotic treatment for bacterial vaginosis. The company revealed that VivaGel BV is now registered in over 50 countries worldwide. Starpharma executed a new licensing deal with ITROM Pharmaceutical Group to commercialize VivaGel BV across 13 countries in the Middle East and North Africa (MENA) region, including Saudi Arabia and the UAE.
- In February 2022, Lupin Pharmaceuticals Inc. received FDA approval for its supplemental New Drug Application (sNDA) to expand SOLOSEC (secnidazole) usage for treating bacterial vaginosis in female patients aged 12 years and older. SOLOSEC maintains its position as the first and only single-dose oral prescription antimicrobial agent approved for both bacterial vaginosis and trichomoniasis treatment. The single-dose formulation provides a complete therapeutic course in one administration, addressing adherence gaps and reducing risk factors associated with these conditions. The approval strengthens Lupin’s women’s health portfolio and provides healthcare professionals with an adolescent treatment option that delivers a full course of therapy in a single dose.
Report Coverage:
The research report offers an in-depth analysis based on Drug Class, Route of Administration, Dosage Form, and Distribution Channel. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Growing focus on lowering recurrence rates will shape future treatment lines.
- Demand for advanced topical formulations will rise among women seeking targeted relief.
- Broader digital consultation access will support faster diagnosis and prescription rates.
- Innovation in microbiome-based therapies will open new product development pathways.
- Online pharmacies will capture higher share due to discreet and convenient access.
- Expanded screening in emerging regions will lift therapy uptake across clinics.
- Drug makers will invest in improved delivery formats that support comfort and adherence.
- Healthcare programs will increase awareness of early symptom identification.
- Combination therapies will gain traction for complex or recurrent BV cases.
- Global partnerships will accelerate development of next-generation antimicrobial options.




