Market Overview:
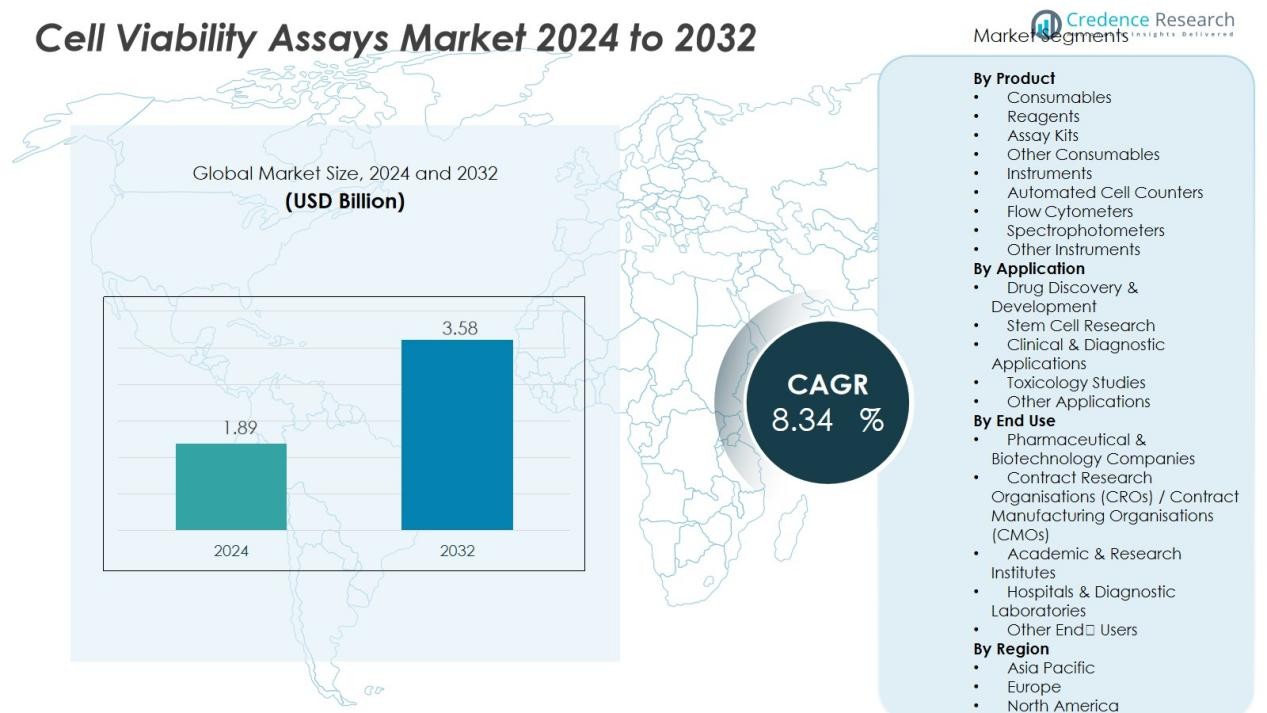
The Cell Viability Assays Market size was valued at USD 1.89 billion in 2024 and is anticipated to reach USD 3.58 billion by 2032, at a CAGR of 8.34 % during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Cell Viability Assays Market Size 2024 |
USD 1.89 Billion |
| Cell Viability Assays Market, CAGR |
8.34% |
| Cell Viability Assays Market Size 2032 |
USD 3.58 Billion |
The market’s growth is primarily driven by the rising need for drug development and cancer research, particularly in oncology and chronic disease treatment. The increasing focus on personalized medicine and regenerative therapies further propels the demand for precise cell viability testing. Additionally, advancements in biotechnology, including high-throughput screening platforms and automated assays, are significantly contributing to market expansion. The increasing number of contract research organizations (CROs) and the growing regulatory requirements for drug testing also act as key drivers.
North America holds the largest share of the cell viability assays market, owing to a robust pharmaceutical and biotechnology industry, coupled with substantial investments in life sciences research. Europe follows closely, driven by stringent regulatory standards and research-driven initiatives. The Asia-Pacific region is anticipated to exhibit the fastest growth, driven by increasing biotech activities in countries like China and India, coupled with the growing adoption of automation in laboratories and clinical trials.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Cell Viability Assays Market was valued at USD 1.89 billion in 2024 and is expected to reach USD 3.58 billion by 2032, growing at a CAGR of 8.34% during the forecast period.
- North America holds the largest share of 35.6% in 2023, driven by a robust pharmaceutical and biotechnology industry, along with substantial investments in life sciences research and advanced lab infrastructure.
- Europe follows closely with a significant share, supported by stringent regulatory standards and strong research initiatives, particularly in countries like Germany, the UK, and France.
- The Asia-Pacific region is the fastest-growing, accounting for 24.5% of the market share in 2023, with rapid biotech developments in China and India, growing clinical trials, and increasing laboratory automation.
- In the product segment, consumables, including reagents and assay kits, dominate the market share, while instruments such as spectrophotometers and flow cytometers show increasing demand with automation trends.
 Market Drivers:
Market Drivers:
Increasing Demand for Drug Discovery and Development
The growing demand for new drug discovery and development is a major driver for the Cell Viability Assays Market. Pharmaceutical companies and research institutions increasingly rely on these assays for screening potential drug candidates, particularly for oncology, cardiovascular, and metabolic diseases. These assays provide critical data on cell proliferation, viability, and cytotoxicity, enabling researchers to assess the effectiveness of drug compounds early in the development process. The ongoing expansion of pharmaceutical pipelines contributes to the rising demand for cell viability assays, particularly in high-throughput formats.
- For instance, Promega launched its CellTiter-Glo 3D Cell Viability Assay, which is optimized for 3D microtissue cultures and is suitable for high-throughput screening in multi-well plates such as 96-well, 384-well, and 1536-well formats
Growth in Personalized Medicine and Regenerative Therapies
The increasing focus on personalized medicine and regenerative therapies further fuels the Cell Viability Assays Market. Tailored treatments for specific patient groups require precise and reliable testing methods to evaluate the effectiveness of new therapies. Cell viability assays play a crucial role in this by allowing researchers to assess cellular responses to various compounds and optimize treatments for individual patients. The rising emphasis on stem cell research and tissue engineering also enhances the need for accurate viability assays to monitor cell health in regenerative medicine applications.
- For instance, Thermo Fisher Scientific’s CellInsight CX7 High-Content Screening Platform enables automated viability screening, supporting numerous personalized medicine programs globally.
Rising Investment in Biotechnology and Research
Substantial investments in biotechnology and life sciences research are driving the demand for cell viability assays. Governments, academic institutions, and private entities are committing increasing resources to advance research in cellular biology and disease mechanisms. This creates a strong demand for advanced testing methods, including cell viability assays, to assess drug efficacy, monitor cell cultures, and validate experimental results. The growing emphasis on developing innovative therapies and the need for robust preclinical testing are expected to sustain this growth trend.
Regulatory Requirements for Safety and Efficacy Testing
The Cell Viability Assays Market is further driven by the increasing regulatory requirements for safety and efficacy testing of new drug candidates. Regulatory bodies across regions are enforcing stringent guidelines for preclinical testing, ensuring that new drugs meet safety standards before clinical trials. Cell viability assays play a critical role in toxicology testing, helping to determine whether drug candidates cause adverse effects on cells. This regulatory emphasis on safety and efficacy increases the demand for reliable, high-quality assays in the drug development process.
Market Trends:
Greater Adoption of Automation and High‑Throughput Technologies
The Cell Viability Assays Market has seen a marked shift towards automated platforms and high‑throughput technologies that reduce manual intervention and deliver faster data. Laboratories now implement robotics and multiplexed workflows to handle large sample volumes while maintaining assay consistency. This transition enables researchers to screen more compounds within the same timeframe and supports drug development pipelines at scale. Demand for instruments that integrate imaging, fluorescence and luminescence read‑outs continues to grow. Suppliers increasingly design systems that streamline workflow and minimise human error, which strengthens assay reproducibility and supports regulatory requirements.
- For instance, Thermo Fisher Scientific’s Invitrogen Attune NxT Flow Cytometer enables analysis of up to 35,000 events per second, allowing users to process large sample batches efficiently and with high reproducibility in screening applications.
Emergence of Label‑Free, Real‑Time and Miniaturised Assay Formats
The market is expanding adoption of label‑free and real‑time monitoring formats that deliver dynamic insights into cell health without destroying samples. It embraces miniaturisation and microfluidic platforms that reduce reagent volumes and increase efficiency of testing workflows. This shift allows researchers to observe cell viability, proliferation or death over time and under more physiologically relevant conditions. The trend also extends to multiplexed read‑outs that combine viability endpoints with other cellular parameters, thereby enhancing data richness from a single experiment. Equipment developers respond by offering more compact, integrated systems suited to both academic and industrial laboratories.
- For instance, Sartorius launched the Octet® R8 system, providing label-free, real-time analysis with throughput of up to 96 samples simultaneously, enabling kinetic measurements at a sensitivity of 0.1 ng/mL for protein quantification.
Market Challenges Analysis:
High Cost and Complex Assay Setup Present Barriers to Growth
The Cell Viability Assays Market faces significant constraints due to high operational and capital costs for advanced assays. Many laboratories require expensive instruments, specialised reagents and strict environmental controls, which raise the barrier to adoption in small‑scale or resource‑limited settings. It often demands extensive training for staff to ensure correct protocol execution and reliable results. Some assay formats involve long incubation periods or complex preparation steps that slow throughput and reduce efficiency. This cost and complexity dynamic may limit uptake among emerging research centres or in regions with constrained funding.
Lack of Standardisation and Reproducibility Undermines Confidence
Variability among assay platforms and protocols undermines reliability of results and hinders broader market adoption. It occurs when different laboratories run similar assays yet obtain divergent results, due to sample handling, cell variability or read‑out differences. This reproducibility challenge prompts concerns among researchers and regulatory bodies about data validity and cross‑study comparisons. The absence of uniform industry standards for assay parameters and calibration further complicates benchmarking across studies. Until reproducibility improves, laboratories may hesitate to invest in new assay formats or shift from established methods.
Market Opportunities:
Expansion into Personalized Medicine and Cell‑Therapy Applications
The Cell Viability Assays Market presents significant opportunity through the growth of personalized medicine and cell‑therapy applications. Researchers increasingly require tools to evaluate individual patient‑derived cells for viability and response to treatments, and such demand drives assay uptake. It offers manufacturers the chance to develop assays tailored for specific cell types or patient cohorts, thereby differentiating products in a crowded field. Growing interest in stem‑cell research and engineered tissues further magnifies this opportunity, creating demand for novel assay formats and reagents. Companies can capture value by offering kits and platforms that support both research and translational pipelines. The shift from one‑size‑fits‑all toward precision assays aligns with emerging therapeutic strategies and broader adoption of personalised approaches.
Global Geographic Expansion and Emerging Market Penetration
Another major opportunity lies in geographic expansion into underserved regions and emerging markets. Many laboratories in Asia‑Pacific, Latin America and Middle East & Africa increasingly invest in life‑sciences infrastructure, creating new markets for viability assays. It allows suppliers to extend their distribution networks, localise products and support regional research initiatives. Partnerships with regional contract research organisations (CROs) and academic institutes can enhance market access and accelerate adoption. With greater global disease burden and rising biotech activity in developing economies, assay providers stand to benefit from first‑mover advantage. Companies that adapt pricing, service and support models to regional needs may gain competitive edge and capture growth outside established Western markets.
Market Segmentation Analysis:
By Product
The product segment within the Cell Viability Assays Market divides into two primary categories: consumables and instruments. The consumables category includes reagents, assay kits, microplates and related items that laboratories use frequently. This segment captures a dominant share because researchers renew supplies continuously and many workflows rely on consumable items for testing viability. The instruments category covers spectrophotometers, cell‑imagers, flow cytometers and other devices required to conduct and read assays. Growth in the instrument segment comes from automation trends and demand for high‑throughput platforms.
- For instance, Revvity’s ViaStain Cell Viability Kits deliver fluorescence-based detection using formulated Calcein AM, Hoechst, and Propidium Iodide reagents optimized for use across their Cellometer automated cell counters and Celigo imaging cytometer platforms.
By Application
The application segment spans drug discovery & development, stem cell research, diagnostics, toxicology and other areas. Drug discovery & development remains the largest sub‑segment, since pharmaceutical firms require viability assays to evaluate candidate compounds and cytotoxicity. Stem cell research registers strong growth due to interest in regenerative medicine and need for viability data in novel therapies. Diagnostic applications gain traction as clinical labs adopt cell viability testing in disease‑monitoring workflows. Each application drives unique demands for assay formats, data throughput, and regulatory compliance.
- For Instance, Quest Diagnostics and other diagnostic laboratories are actively leveraging advancements in digital pathology and artificial intelligence (AI) to improve the efficiency and accuracy of testing, particularly in the field of oncology.
By End‑User
End‑users of the Cell Viability Assays Market include pharmaceutical & biotechnology companies, contract research organisations (CROs) & contract manufacturing organisations (CMOs), academic & research institutes, and diagnostic laboratories. Pharmaceutical & biotechnology companies lead market uptake because they perform extensive in‑house R&D and need robust viability testing services. CROs/CMOs benefit from outsourcing trends and expanding screening services, so they represent a fast‑growing segment. Academic & research institutes drive demand for basic research, novel assay development and training applications. Diagnostic laboratories offer opportunity by integrating viability assays into clinical workflows, though they face higher regulatory and cost constraints.
Segmentations:
By Product
- Consumables
- Reagents
- Assay Kits
- Other Consumables
- Instruments
- Automated Cell Counters
- Flow Cytometers
- Spectrophotometers
- Other Instruments
By Application
- Drug Discovery & Development
- Stem Cell Research
- Clinical & Diagnostic Applications
- Toxicology Studies
- Other Applications
By End‑User
- Pharmaceutical & Biotechnology Companies
- Contract Research Organisations (CROs) / Contract Manufacturing Organisations (CMOs)
- Academic & Research Institutes
- Hospitals & Diagnostic Laboratories
- Other End‑Users
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America Region
North America held a market share of 35.6% in the region for the Cell Viability Assays Market in 2023. The region benefits from a mature pharmaceutical and biotechnology ecosystem, strong funding for life‑sciences research and wide adoption of advanced laboratory infrastructure. It drives demand for cell viability assays primarily through drug development, toxicology testing and stem cell applications. The presence of major assay‑reagent and instrument manufacturers further supports local production, distribution and innovation. Research organisations and contract research organisations (CROs) contribute significantly to volume demand, especially in the United States and Canada. Regulatory frameworks that emphasise cytotoxicity and viability testing also encourage assay adoption. Ongoing collaborations between industry and academic institutes reinforce the region’s leadership.
Asia‑Pacific Region
Asia‑Pacific accounted for a market share of 24.5% in 2023 within the Cell Viability Assays Market. Rapid growth in pharmaceutical R&D, growing clinical‑trial volumes and increasing cellular‑biology research in China, India and Southeast Asia drive the region’s expansion. It shows the fastest growth trajectory, supported by emerging biotech hubs, rising government investments and increasing laboratory automation. Many local laboratories upgrade from basic equipment to high‑throughput platforms, which raises demand for viability assays. The rise of domestic CROs and partnerships with global vendors also strengthens market penetration. While infrastructure still trails mature regions, the improvement pace offers a strong opportunity for assay‑providers.
Europe, Latin America & Middle East / Africa Regions
Europe achieved a share second only to North America, while Latin America and the Middle East & Africa combined captured smaller slices of the global market. In Europe, high research intensity, favourable funding and regulatory drivers contribute to steady demand. It sees strong uptake in consumables and instruments from academic and industrial users across Germany, UK, France and the Nordics. Latin America shows growth propelled by expansion of biotech clusters in Brazil and Mexico, yet budget and infrastructure constraints limit adoption speed. The Middle East & Africa region exhibits nascent demand; growing hospital and research‑lab investments signal emerging potential though scale remains limited. Each of these regions requires tailored market‑entry strategies and local servicing models to build share.
Key Player Analysis:
- Thermo Fisher Scientific, Inc.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Merck KGaA
- BD
- PerkinElmer Inc.
- Promega Corporation
- Biotium
- Creative Bioarray
- Abcam plc
- Charles River Laboratories
Competitive Analysis:
The competitive landscape of the Cell Viability Assays Market underscores the presence of major players vying for leadership and differentiation. Key firms in this domain include Thermo Fisher Scientific, Inc., Agilent Technologies, Inc., Bio‑Rad Laboratories, Inc., Merck KGaA and Becton, Dickinson and Company.
Thermo Fisher Scientific leads this market segment through its broad portfolio of cell‑viability reagents and high‑throughput instrumentation. It capitalises on strong global distribution channels and invests in innovation to strengthen its position. Agilent Technologies leverages its instrumentation heritage and accommodates cell viability workflows via integrated platforms. Bio‑Rad Laboratories emphasises both consumables and instrument systems tailored to life‑science research, while Merck KGaA supplies reagents and kits focussed on assay performance. Becton, Dickinson and Company rounds out this group through its clinical‑lab and research‑lab product lines that interface with viability testing.
Competition among these firms revolves around product launches, partnerships and geographic expansion. They actively target mid‑to‑large laboratories with fully automated assay solutions and offer service support to enhance customer workflow. They confront challenges such as the need for cost‑effective models, local regulatory compliance and reproducibility assurance. Suppliers that deliver flexible assay formats, seamless integration with downstream analytics and adaptable pricing will gain advantage.
Recent Developments:
- In September 2025, Thermo Fisher Scientific completed the acquisition of Sanofi’s sterile fill-finish and packaging facility in Ridgefield, New Jersey, expanding its strategic partnership to increase U.S. manufacturing capacity.
- In June 2025, Agilent Technologies entered a strategic collaboration with SeqOne to enhance liquid biopsy data analysis for oncology research.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage:
The research report offers an in-depth analysis based on Product, Application, End‑User and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The future of the Cell Viability Assays Market is poised for robust growth, driven by advancements in biotechnology and increasing demand for personalized medicine.
- The demand for cell viability assays will expand as drug discovery and development efforts intensify.
- Technological advancements in automation and high‑throughput screening platforms will enhance assay efficiency and accuracy.
- Ongoing research in regenerative medicine and stem cell therapies will further drive the need for reliable viability testing.
- The integration of AI and machine learning in data analysis will accelerate the adoption of more sophisticated assay methods.
- Increasing adoption of label‑free and real‑time assay technologies will reshape research workflows and reduce operational costs.
- Regulatory pressure to adopt more stringent safety testing standards will drive the need for reliable cell viability assays in preclinical studies.
- Emerging biotech hubs in Asia‑Pacific and Latin America will see increased demand for cell viability assays as local research capabilities expand.
- Collaborations between academic institutions and biotech firms will stimulate innovation in assay technologies, making them more versatile.
- Growing investments in research funding, particularly in oncology and chronic disease treatments, will sustain market growth and fuel product innovation.

 Market Drivers:
Market Drivers:

