Market Overview
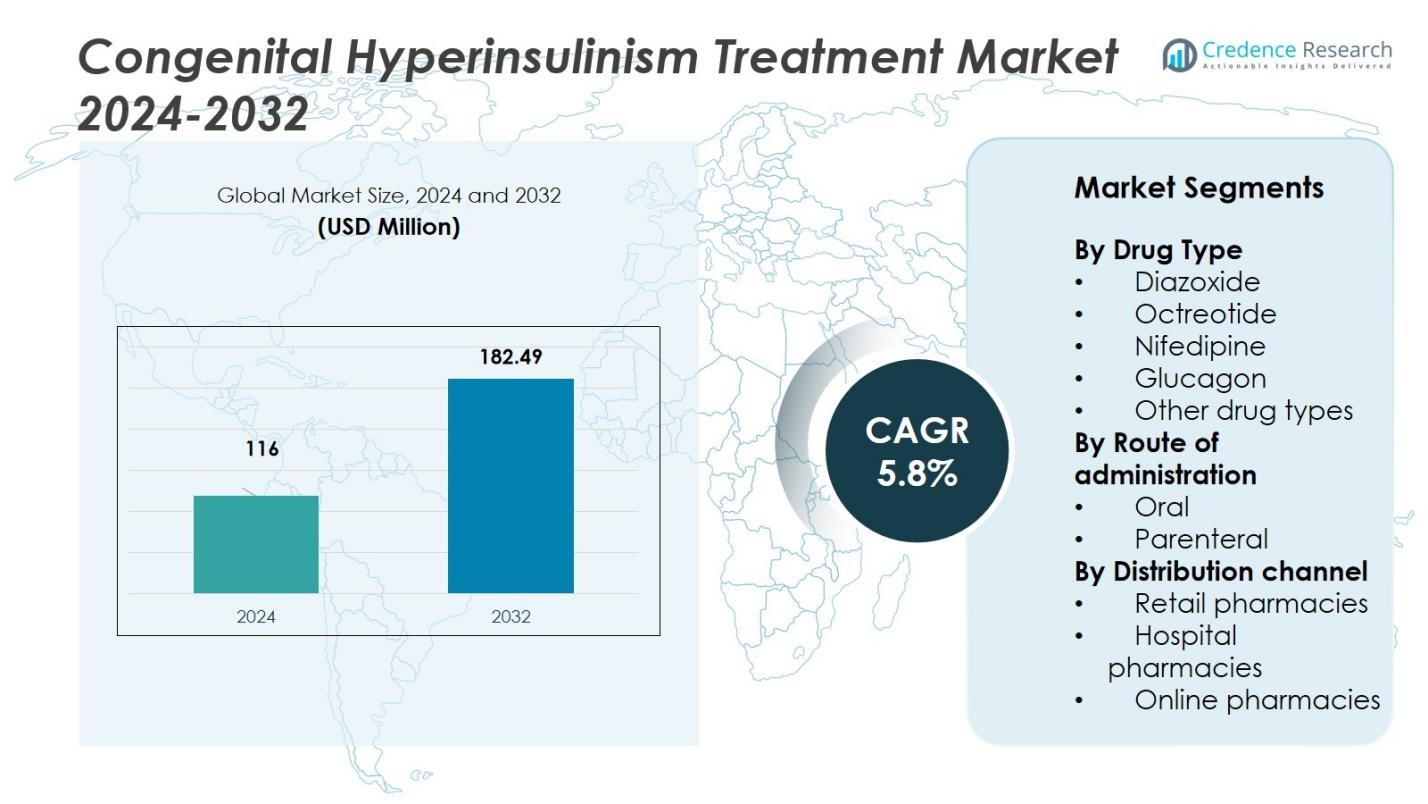
The Congenital Hyperinsulinism Treatment Market size was valued at USD 116 million in 2024 and is anticipated to reach USD 182.49 million by 2032, at a CAGR of 5.8% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Congenital Hyperinsulinism Treatment Market Size 2024 |
USD 116 Million |
| Congenital Hyperinsulinism Treatment Market, CAGR |
5.8% |
| Congenital Hyperinsulinism Treatment Market Size 2032 |
USD 182.49 Million |
The Congenital Hyperinsulinism Treatment Market is driven by key players such as Novartis International AG, Ipsen S.A., Pfizer Inc., and Teva Pharmaceutical Industries Ltd. These companies are at the forefront of developing specialized treatments and therapies for congenital hyperinsulinism, focusing on innovative drug formulations and improving patient outcomes. Novartis and Ipsen lead with established products like Diazoxide, while Pfizer and Teva are expanding their portfolios to include newer therapies aimed at managing the disease. North America holds the largest market share, accounting for 61.3% of the global market, supported by strong healthcare infrastructure, research investments, and favorable regulatory frameworks. Europe follows with a 22.4% market share, driven by advanced diagnostic capabilities and the adoption of rare disease treatment policies. Meanwhile, emerging regions, such as Asia-Pacific, are poised for rapid growth due to increasing awareness, healthcare improvements, and rising demand for effective treatment options.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Congenital Hyperinsulinism Treatment Market was valued at USD 116 million in 2024 and is expected to reach USD 182.49 million by 2032, growing at a CAGR of 5.8%.
- The increasing prevalence of congenital hyperinsulinism and rising awareness are driving the market demand for effective treatments.
- The shift towards oral drug formulations is a key trend, enhancing patient compliance and driving market growth, especially in pediatric treatments.
- Market restraints include the high cost of therapies and limited awareness in low-income regions, hindering wider adoption of treatments.
- North America leads the market with a 61.3% share, followed by Europe at 22.4%, with Asia-Pacific being the fastest-growing region due to improved healthcare access and rising diagnoses. The drug type segment, particularly Diazoxide, dominates the market with a 45% share.
Market Segmentation Analysis:
By Drug Type
The drug type segment in the Congenital Hyperinsulinism Treatment Market includes Diazoxide, Octreotide, Nifedipine, Glucagon, and other drug types. Diazoxide dominates the segment, holding approximately 45% of the market share due to its long-standing use as a first-line treatment for managing congenital hyperinsulinism. The effectiveness of Diazoxide in controlling hypoglycemic episodes makes it the preferred choice among healthcare providers. The market is driven by the increasing prevalence of congenital hyperinsulinism and the growing adoption of Diazoxide as the standard therapy, contributing to its leading position in the segment.
- For instance, Diazoxide is marketed under the brand name Proglycem® and is widely used to control hypoglycemia by blocking insulin release; it is the only FDA-approved drug for this condition in children, with extensive use in hospitals and pediatric care settings.
By Route of Administration
The Congenital Hyperinsulinism Treatment Market is divided into oral and parenteral routes of administration. The oral route leads the market, holding a market share of around 60%, as it is preferred for its ease of administration and patient compliance. Drugs like Diazoxide and Nifedipine are commonly administered orally, which is especially beneficial for pediatric patients. The oral administration segment is driven by the convenience it offers, improving patient adherence to long-term treatment regimens and contributing to its dominance in the market.
- For instance, Diazoxide is the only FDA-approved oral medication for treating hyperinsulinism in neonates and is widely used to manage persistent hypoglycemia by inhibiting insulin secretion from pancreatic beta cells.
By Distribution Channel
The distribution channel segment is categorized into retail pharmacies, hospital pharmacies, and online pharmacies. Hospital pharmacies lead the market, commanding approximately 55% of the market share, driven by their key role in providing specialized treatments to hospitalized patients. Hospitals often have a higher demand for critical care medications, including those used in the treatment of congenital hyperinsulinism. The hospital pharmacy segment benefits from strong relationships with healthcare providers and hospitals, contributing to its market leadership and consistent growth.
Key Growth Drivers
Increasing Prevalence of Congenital Hyperinsulinism
The growing incidence of congenital hyperinsulinism worldwide is a major driver for the treatment market. With rising awareness about the condition and advancements in diagnostics, more cases are being identified. This contributes to a higher demand for effective treatments such as Diazoxide and other therapeutic drugs. The increasing prevalence, particularly in neonates and infants, fuels market growth as healthcare providers focus on early intervention and improved management options to control hypoglycemia, boosting the demand for medications.
- For instance, congenital hyperinsulinism occurs in approximately 1 in 25,000 to 1 in 50,000 live births, with about 60% of affected babies diagnosed within the first month of life, highlighting the critical need for early diagnosis and intervention.
Advancements in Drug Development
Recent advancements in drug development are significantly contributing to the growth of the Congenital Hyperinsulinism Treatment Market. The development of new therapies, such as long-acting formulations and combination treatments, provides better treatment outcomes for patients. These innovations improve patient compliance and reduce the frequency of hospital visits, which enhances the overall treatment experience. Additionally, improved safety profiles and targeted therapies are expected to further expand the market, as they offer better control of blood sugar levels without major side effects.
- For instance, Novartis has developed a long-acting release (LAR) octreotide formulation, which replaces multiple daily injections with a single monthly intramuscular injection.
Rising Awareness and Diagnosis
Increased awareness about congenital hyperinsulinism among healthcare professionals and the general public is driving market growth. Better diagnostic tools and early detection allow for quicker and more accurate identification of the condition, which leads to timely treatment initiation. Enhanced understanding of the disease also prompts earlier interventions, reducing the risks associated with untreated hyperinsulinism. As a result, more patients are seeking medical attention, which leads to increased demand for treatment options and medications.
Key Trends & Opportunities
Focus on Pediatric Patient Population
A significant opportunity in the Congenital Hyperinsulinism Treatment Market lies in the pediatric population, especially neonates and infants. As congenital hyperinsulinism predominantly affects children, there is a growing focus on developing pediatric-specific formulations and dosages. These treatments are designed to be safer and more effective for young patients, with minimal side effects. Pharmaceutical companies are increasingly targeting this demographic, offering tailored therapies that are likely to drive market expansion and address an unmet need in pediatric endocrinology.
- For instance, Hanmi Pharmaceutical is developing efpegerglucagon (HM15136), a novel once-weekly injectable treatment currently in Phase 2 trials, targeting pediatric patients with congenital hyperinsulinism to reduce hypoglycemia and improve treatment adherence.
Shift Towards Oral Medications
The shift towards oral medications presents an important opportunity in the Congenital Hyperinsulinism Treatment Market. Oral formulations, such as Diazoxide, are preferred due to their ease of administration, improving patient compliance. The trend is gaining momentum as it reduces the burden on caregivers and healthcare providers, particularly for pediatric patients. The demand for oral drug formulations is expected to rise as they offer a more convenient alternative to injectable therapies, enhancing the patient experience and making treatment more manageable for both children and adults.
- For instance, PROGLYCEM® (oral Diazoxide suspension) offers a liquid formulation widely used despite challenges of availability and cost, providing a convenient alternative to injectable treatments
Key Challenges
High Cost of Treatment
One of the significant challenges facing the Congenital Hyperinsulinism Treatment Market is the high cost of treatment. The advanced therapies and medications required to manage congenital hyperinsulinism, especially for severe cases, can be expensive. These costs put a financial strain on patients, caregivers, and healthcare systems, limiting access to effective treatments in low-income regions. Additionally, the long-term nature of the treatment regimen contributes to the cumulative financial burden, posing a challenge to widespread adoption and affordability.
Limited Awareness in Low-Income Regions
Limited awareness and access to healthcare services in low-income regions remain a significant challenge. While congenital hyperinsulinism is a rare condition, it is especially underdiagnosed and untreated in resource-poor areas due to a lack of awareness among healthcare professionals. This lack of early diagnosis and proper treatment can lead to complications, further hindering the growth of the market in these regions. Expanding awareness and improving healthcare infrastructure are critical to addressing this gap and increasing market penetration globally.
Regional Analysis
North America
North America holds the largest regional share in the Congenital Hyperinsulinism Treatment Market, accounting for 61.3% of the global market. The region benefits from advanced healthcare infrastructure, strong research and development investments, and favorable regulatory pathways that support orphan drug approvals and rare disease care. Early diagnosis and availability of specialized centers drive the uptake of approved treatments, boosting market growth. Major players target this market to capture the high per-patient revenue and benefit from the better reimbursement environment in the region.
Europe
Europe accounts for 22.4% of the global congenital hyperinsulinism treatment market. Growth in this region is supported by well-established rare disease networks, increasing awareness of pediatric endocrinology disorders, and expanding access to diagnostic and treatment facilities. Regulatory incentives and orphan-drug designations further strengthen market uptake. European demand for treatment options is steadily increasing due to improved patient identification, broader reimbursement coverage, and the availability of specialized care, making it a key player in the global market.
Asia-Pacific
Asia-Pacific is the fastest-growing region in the congenital hyperinsulinism treatment market, holding a market share of 9.5%. Growth is driven by rising awareness of rare pediatric disorders, improving healthcare access in key countries such as India and China, and growing investment in pediatric endocrinology infrastructure. Emerging markets are witnessing increased diagnoses of congenital hyperinsulinism, which expands the treatment base and presents a strong opportunity for market expansion. This region is expected to see a rapid increase in treatment adoption in the coming years.
Latin America
Latin America holds a smaller share of the global congenital hyperinsulinism treatment market, with a market share of 4.2%. However, the region is witnessing growth driven by expanding healthcare infrastructure, increased government focus on rare disease care, and improved access to specialized diagnostics. The market in Latin America is expected to develop further as healthcare payer systems widen coverage, and as treatment awareness among clinicians and caregivers improves, contributing to the region’s gradual market expansion.
Middle East & Africa
The Middle East & Africa region represents the smallest share in the congenital hyperinsulinism treatment market, with a market share of 2.6%. Limited diagnostic infrastructure and lower treatment access currently restrict market size. However, rising awareness of the incidence of congenital hyperinsulinism, philanthropic support for rare diseases, and expanding specialty care clinics are gradually improving the market outlook. Increased regional investment and improved access to orphan therapies may boost the region’s market share in the future.
Market Segmentations:
By Drug Type
- Diazoxide
- Octreotide
- Nifedipine
- Glucagon
- Other drug types
By Route of administration
By Distribution channel
- Retail pharmacies
- Hospital pharmacies
- Online pharmacies
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The Congenital Hyperinsulinism Treatment Market is highly competitive, with key players such as Novartis International AG, Ipsen S.A., Pfizer Inc., and Teva Pharmaceutical Industries Ltd. leading the market. These companies have established strong market positions through the development of specialized treatments, strategic partnerships, and a focus on expanding their product portfolios. The competitive landscape is shaped by ongoing research and development activities aimed at improving the efficacy and safety of treatments, such as Diazoxide and other emerging therapies. Companies are increasingly focusing on pediatric formulations, as congenital hyperinsulinism predominantly affects infants and children. Additionally, the market is witnessing growth in both developed and emerging markets, with players strengthening their distribution networks to increase accessibility to their products. Competitive strategies also include securing regulatory approvals, focusing on orphan drug designations, and offering cost-effective treatment options to address the high demand for congenital hyperinsulinism therapies worldwide.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Teva Pharmaceutical Industries Ltd.
- Mylan N.V.
- Viatris Inc.
- Sun Pharmaceutical Industries Ltd.
- Biocon Ltd.
- Novartis International AG
- Ipsen S.A.
- Pfizer Inc.
- Hetero Laboratories Ltd.
- Sandoz International GmbH
Recent Developments
- In July 2024, Amylyx Pharmaceuticals, Inc. acquired Avexitide, a GLP-1 receptor antagonist from Eiger BioPharmaceuticals, Inc. This drug is being developed for hyperinsulinemic hypoglycemia, including CHI.
- In June 2023, Zealand Pharma A/S submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for Dasiglucagon, a treatment for CHI.
- In February 2023, Rhythm Pharmaceuticals, Inc. acquired Xinvento BV, a Netherlands-based biotech firm focused on therapies for CHI.
Report Coverage
The research report offers an in-depth analysis based on Drug Type, Route of Administartion, Distribution Channel and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The congenital hyperinsulinism treatment market will continue to grow with increasing awareness and early diagnosis.
- Advances in pediatric-specific formulations and treatments will drive market demand.
- The adoption of oral treatments over injectable therapies will rise, improving patient compliance.
- Emerging markets will witness rapid market expansion due to improved healthcare infrastructure.
- Companies will focus on developing safer, more effective therapies with fewer side effects.
- Strategic partnerships and collaborations will intensify, enhancing research and development efforts.
- The demand for long-acting treatments and combination therapies will increase.
- More regulatory incentives for orphan drugs will foster market growth.
- Cost-effective treatment solutions will gain traction, especially in emerging economies.
- Ongoing clinical trials and innovations will expand the available treatment options for congenital hyperinsulinism.




