Market Overview:
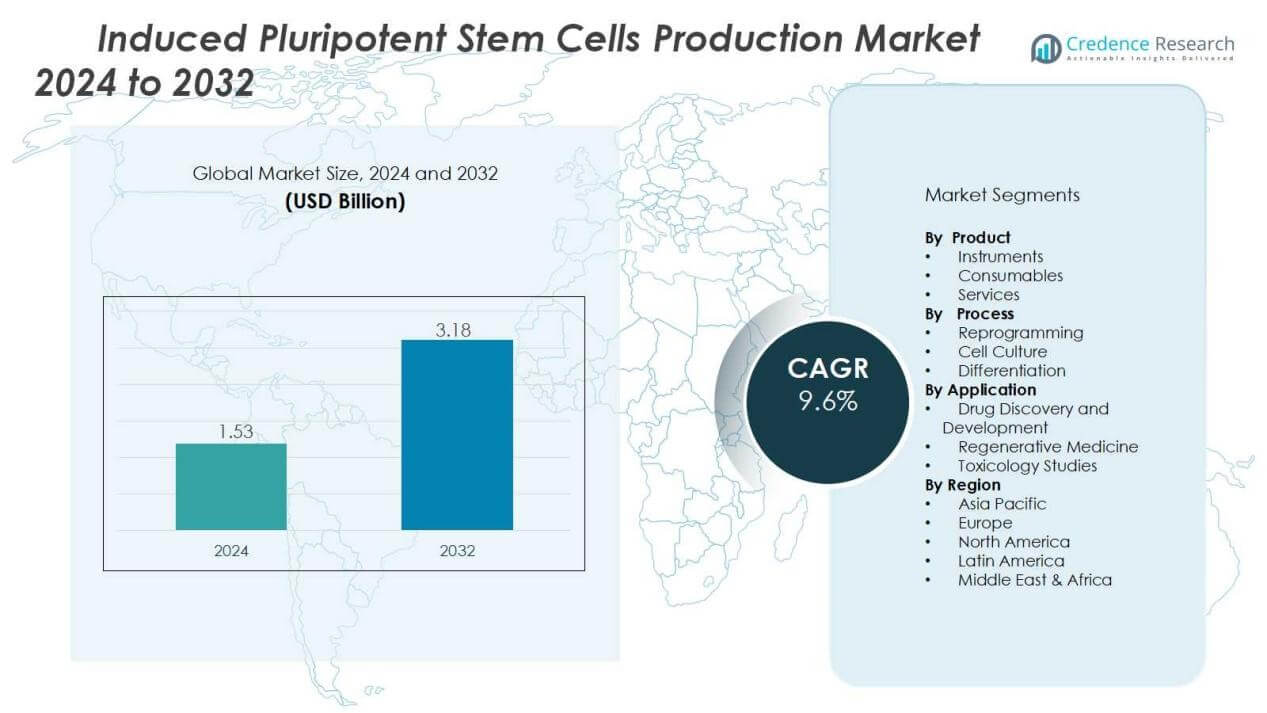
The induced pluripotent stem cells production market size was valued at USD 1.53 billion in 2024 and is anticipated to reach USD 3.18 billion by 2032, at a CAGR of 9.6% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Induced Pluripotent Stem Cells Production Market Size 2024 |
USD 1.53 Billion |
| Induced Pluripotent Stem Cells Production Market, CAGR |
9.6% |
| Induced Pluripotent Stem Cells Production Market Size 2032 |
USD 3.18 Billion |
Market growth is driven by several key factors. Increasing prevalence of chronic and genetic disorders is fueling demand for cell-based therapies and personalized medicine. Continuous technological progress in genome editing, automation, and culture systems enhances efficiency and lowers production costs, making iPSCs more accessible. Growing partnerships between biopharmaceutical companies and research institutes, coupled with regulatory support for cell-based research, are further propelling market expansion.
Regionally, North America holds a dominant share, supported by advanced healthcare infrastructure, strong research funding, and presence of leading biotechnology firms. Europe follows closely, with supportive government policies and widespread adoption of stem cell research in academic centers. Asia-Pacific is projected to witness the fastest growth, driven by large patient populations, rising healthcare expenditure, and increasing investment in regenerative medicine, particularly in countries such as China, Japan, and South Korea.
Market Insights:
- The induced pluripotent stem cells production market was valued at USD 1.53 billion in 2024 and is expected to reach USD 3.18 billion by 2032, growing at a CAGR of 9.6% during 2024–2032.
- Rising demand for regenerative medicine strengthens adoption, as iPSCs provide patient-specific therapies for tissue repair and organ regeneration.
- Drug discovery remains a leading application, with iPSC-derived models improving disease understanding, drug efficacy testing, and personalized medicine strategies.
- Technological advancements in genome editing, automation, and bioreactor-based culture systems are reducing costs and improving scalability.
- High production costs and regulatory hurdles continue to challenge broader adoption, particularly in emerging markets.
- North America leads with 42% share, driven by advanced infrastructure, research funding, and widespread clinical integration.
- Asia-Pacific, holding 22% share, is projected to grow fastest due to high investment, large patient populations, and government support for regenerative medicine.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Demand for Regenerative Medicine and Cell-Based Therapies:
The induced pluripotent stem cells production market benefits from growing applications in regenerative medicine. iPSCs offer patient-specific solutions for tissue repair, organ regeneration, and disease modeling. This capability reduces the risk of immune rejection and enhances treatment outcomes. Rising prevalence of chronic diseases such as cardiovascular disorders and diabetes further strengthens demand for iPSC-based therapies. It positions the technology as a cornerstone of next-generation healthcare.
- In July 2025, REPROCELL announced the submission of a U.S. FDA Drug Master File for its StemRNA Clinical iPSC Seed Stock Clones, offering ethically sourced, regulatory-compliant, virus-free, clinical-grade iPSC lines from U.S. and Japan facilities.
Growing Role in Drug Discovery and Personalized Medicine:
Pharmaceutical companies are adopting iPSCs to accelerate drug discovery and development. iPSC-derived models provide accurate platforms for studying disease mechanisms and testing drug efficacy. These models enable safer clinical trials by predicting toxicity and patient-specific responses. Personalized medicine strategies rely heavily on such platforms to tailor treatments. The induced pluripotent stem cells production market gains momentum through expanding industry collaborations.
- For Instance, FUJIFILM Corporation announced a $200 million investment in December 2023 to expand cell therapy development and manufacturing across two U.S. subsidiaries, including an expansion of FUJIFILM Cellular Dynamics Inc.’s new headquarters in Madison, Wisconsin.
Technological Advancements Enhancing Efficiency and Scalability:
Continuous progress in reprogramming techniques and culture systems drives efficiency in iPSC production. Automation, bioreactor technologies, and gene-editing tools improve scalability and reproducibility. Such innovations reduce costs and make production processes more reliable. Companies and research institutes are investing in integrated platforms to meet commercial demand. It strengthens the potential of iPSCs in both clinical and industrial applications.
Supportive Government Funding and Academic Research Initiatives:
Public funding and policy support remain crucial drivers of market growth. Governments in North America, Europe, and Asia-Pacific are promoting large-scale stem cell research projects. Academic institutions are advancing disease models and therapy development using iPSCs. This ecosystem fosters collaboration between academia and industry, accelerating translation into clinical practice. The induced pluripotent stem cells production market continues to expand under this supportive research environment.
 Market Trends:
Market Trends:
Integration of iPSCs with Gene Editing and Advanced Bioprocessing Technologies:
The induced pluripotent stem cells production market is witnessing a strong trend toward integrating iPSCs with CRISPR and other advanced gene-editing tools. This combination enhances precision in disease modeling and supports development of targeted therapies. Bioprocessing innovations such as bioreactor-based culture systems and automated platforms are further improving scalability and efficiency. These advancements reduce manual intervention and lower risks of variability, making large-scale production feasible. Pharmaceutical and biotechnology companies are increasingly investing in standardized protocols to ensure reproducibility and regulatory compliance. It strengthens the role of iPSCs in both commercial and clinical settings, opening pathways for broader adoption.
- For instance, Carr Biosystems has demonstrated that integrating single-use tubular bowl centrifugation with Vertical-Wheel bioreactors preserves high iPSC recovery and viability across scalable working volumes from 60 mL to 80 L, enabling consistent expansion suitable for industrial production.
Expansion of iPSC Applications in Personalized Medicine and Drug Screening:
Personalized medicine is driving new applications for iPSCs in tailoring treatments to patient-specific genetic profiles. iPSC-derived models provide valuable insights into disease progression and drug responses, reducing failures in clinical trials. This trend supports the pharmaceutical industry’s focus on precision medicine and cost-effective drug development. The induced pluripotent stem cells production market benefits from expanding collaborations between research institutions and biopharma companies. Growth in organoid and tissue engineering research highlights the versatility of iPSCs in creating complex human models. It positions iPSCs as an essential tool for future healthcare innovations, from advanced diagnostics to regenerative solutions.
- For instance, Takara Bio’s Cellartis Definitive Endoderm Differentiation Kit generated over 80 percent SOX17-positive endodermal cells within five days, demonstrating robust, high-efficiency iPSC differentiation for drug toxicity screening
Market Challenges Analysis:
High Production Costs and Technical Complexities in Scaling:
The induced pluripotent stem cells production market faces significant cost-related challenges due to complex reprogramming processes and expensive culture media. Scaling production to meet commercial demand requires advanced infrastructure and skilled expertise, creating financial barriers for smaller players. Variability in reprogramming efficiency also limits reproducibility, raising concerns about quality control. Automation and bioreactor technologies help address scalability, but they involve high capital investment. Regulatory authorities demand strict compliance, further adding to operational costs. It restricts broader adoption across emerging markets where resources remain limited.
Ethical Concerns and Regulatory Barriers in Clinical Translation:
Ethical considerations surrounding genetic manipulation and patient-derived samples pose hurdles to global acceptance. Regulatory agencies enforce rigorous approval processes, slowing clinical translation of iPSC-based therapies. Concerns about tumorigenicity and genomic instability remain unresolved, affecting confidence in long-term safety. Researchers must demonstrate consistent results to satisfy safety and efficacy requirements, which delays commercialization. Limited harmonization of guidelines across regions complicates international collaborations and market expansion. The induced pluripotent stem cells production market must address these issues to achieve sustainable growth and clinical acceptance.
Market Opportunities:
Expanding Applications in Regenerative Medicine and Disease Modeling:
The induced pluripotent stem cells production market offers significant opportunities through its growing role in regenerative medicine. iPSCs enable patient-specific therapies that can repair tissues and restore organ function, addressing unmet medical needs. Their use in modeling complex diseases also supports pharmaceutical innovation by improving drug screening and reducing trial failures. Rising demand for organoids and tissue-engineered models expands applications across oncology, neurology, and cardiology. Academic and clinical partnerships are accelerating development pipelines, creating favorable conditions for commercialization. It positions iPSCs as a cornerstone technology in advancing precision healthcare.
Emerging Potential in Personalized Medicine and Global Collaborations:
Personalized medicine strategies create strong demand for iPSCs in tailoring therapies to individual patients. iPSC-derived cell lines allow deeper understanding of genetic variations, paving the way for more targeted treatments. The induced pluripotent stem cells production market benefits from growing collaborations between biotech companies, research institutes, and healthcare providers. Expanding investments in Asia-Pacific and Europe are opening new avenues for clinical research and therapy development. Advancements in automation and bioprocessing will lower costs and enhance scalability, supporting broader adoption. It presents long-term opportunities for stakeholders to capture value across diverse healthcare and pharmaceutical domains.
Market Segmentation Analysis:
By Product:
The induced pluripotent stem cells production market is segmented into instruments, consumables, and services. Consumables hold a significant share due to recurring demand for reagents, media, and culture kits in both research and clinical settings. Instruments such as cell culture systems and bioreactors are gaining traction as automation drives efficiency and scalability. Services, including contract manufacturing and specialized iPSC generation, are expanding with growing partnerships between research institutes and industry. It highlights strong dependence on consumables while instruments and services grow steadily.
- For instance, the CellXpress.ai Automated Cell Culture System by Molecular Devices automates media exchanges every 24 hours and delivers imaging-based monitoring every 12 hours, enabling labs to achieve up to 70% cell confluency for automated passaging in six-well plate formats, significantly optimizing large-scale iPSC expansion workflows.
By Process:
Segmentation by process includes reprogramming, cell culture, and differentiation. Reprogramming dominates due to its role in generating patient-specific iPSCs through advanced genetic and non-genetic methods. Cell culture processes are expanding with innovations in bioreactors that ensure reproducibility and reduce variability. Differentiation remains a critical segment as iPSCs are directed into specialized cell types for disease modeling and therapeutic use. It reflects a balanced focus on all processes to support both clinical and commercial outcomes.
For Instance, REPROCELL states its StemRNA™ 3rd Generation method achieves 2.00–4.00% reprogramming efficiency, making it at least 50 times more effective than its Epi5 episomal method, which has an efficiency of 0.03–0.04%.
By Application:
Applications are divided into drug discovery and development, regenerative medicine, and toxicology studies. Drug discovery leads due to its use of iPSC-derived models for testing efficacy and reducing trial risks. Regenerative medicine shows rapid growth with its ability to address unmet needs in organ repair and tissue regeneration. Toxicology studies are gaining relevance by providing safer alternatives to animal testing. It positions iPSCs as a versatile tool across diverse applications in healthcare and pharmaceuticals.
Segmentations:
By Product:
- Instruments
- Consumables
- Services
By Process:
- Reprogramming
- Cell Culture
- Differentiation
By Application:
- Drug Discovery and Development
- Regenerative Medicine
- Toxicology Studies
By Region:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
North America:
North America holds 42% market share in the induced pluripotent stem cells production market, making it the leading region. The United States dominates due to advanced research infrastructure, significant funding, and strong presence of biopharmaceutical companies. Government-backed initiatives and favorable policies promote iPSC-based clinical trials and translational research. Academic institutions and private organizations collaborate extensively, driving innovation in regenerative medicine and drug discovery. High prevalence of chronic diseases further fuels demand for cell-based therapies. It continues to strengthen the region’s leadership through technological advancements and established regulatory frameworks.
Europe:
Europe accounts for 30% market share, positioning it as the second-largest regional market. Countries such as Germany, the United Kingdom, and France lead adoption through supportive regulatory policies and public research programs. The European Medicines Agency provides structured guidelines, encouraging safe integration of iPSCs into clinical applications. High investment in personalized medicine and strong focus on disease modeling drive regional momentum. Collaborative projects between universities and biopharma companies foster scalable innovations. It leverages its advanced healthcare systems and strict quality standards to expand iPSC applications across multiple therapeutic areas.
Asia-Pacific:
Asia-Pacific holds 22% market share, supported by rapid advancements in stem cell research and healthcare infrastructure. China, Japan, and South Korea lead regional progress through high investment and strong government support for regenerative medicine. Large patient populations create high demand for personalized therapies and innovative drug testing platforms. Expanding clinical trials and partnerships with global biopharma players enhance regional competitiveness. Rising healthcare expenditure and focus on biotechnology strengthen long-term adoption of iPSCs. It is projected to achieve the fastest growth rate, reshaping the global landscape for stem cell-based innovations.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Lonza
- Evotec
- Axol Bioscience Ltd.
- REPROCELL Inc.
- Merck KGaA
- Hitachi, Ltd.
- Fate Therapeutics
- StemCellsFactory III
- Thermo Fisher Scientific, Inc.
- Applied StemCells, Inc.
Competitive Analysis:
The induced pluripotent stem cells production market features a competitive landscape shaped by global biotechnology and pharmaceutical leaders. Key players include Lonza, Evotec, Axol Bioscience Ltd., REPROCELL Inc., Merck KGaA, and Hitachi, Ltd., each contributing through product innovation and strategic partnerships. Companies focus on advancing reprogramming methods, improving culture systems, and developing scalable solutions to support both research and clinical applications. Strong emphasis on automation and bioreactor technologies positions market leaders to meet rising demand for reproducibility and efficiency. Partnerships between industry and academic institutions foster innovation and expand therapeutic pipelines. It continues to evolve through mergers, collaborations, and investments aimed at expanding service portfolios and geographic presence. Competitive strategies highlight an industry-wide push toward strengthening regulatory compliance and accelerating the clinical translation of iPSC-based therapies.
Recent Developments:
- In May 2025, Lonza published 2024 comparative financials under the new One Lonza organizational structure, effective from April 2025, and consisting of three CDMO business platforms.
- In October 2024, Lonza extended a long-term collaboration with a major pharmaceutical partner for the integrated commercial supply of antibody-drug conjugates, including constructing new customer-dedicated bioconjugation capacity in Visp (CH).
Report Coverage:
The research report offers an in-depth analysis based on Product, Process, Application and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The induced pluripotent stem cells production market will see broader adoption in regenerative medicine to address unmet clinical needs.
- It will expand in drug discovery as iPSC-derived models reduce trial failures and improve accuracy.
- Demand will grow in personalized medicine, with iPSCs enabling patient-specific therapies and diagnostics.
- Integration of CRISPR and advanced gene-editing tools will strengthen applications in disease modeling.
- Automation and bioreactor innovations will reduce production costs and support large-scale deployment.
- Global collaborations between academic institutions and biopharma companies will accelerate clinical translation.
- Asia-Pacific will play a vital role in market expansion due to high investment and patient pools.
- Regulatory frameworks will evolve to support safe commercialization of iPSC-based therapies worldwide.
- Rising use of organoids and tissue engineering will create new opportunities across multiple therapeutic areas.
- It will remain a cornerstone technology in precision healthcare, reshaping treatment approaches and driving innovation.

 Market Trends:
Market Trends:

