Market Overview
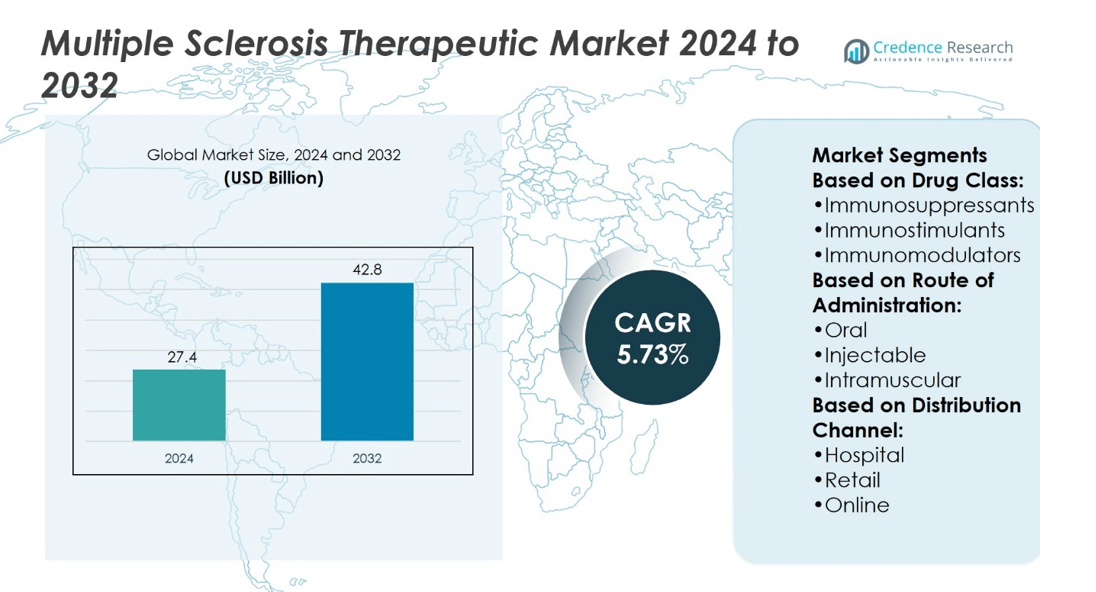
Multiple Sclerosis Therapeutic Market size was valued at USD 27.4 billion in 2024 and is anticipated to reach USD 42.8 billion by 2032, at a CAGR of 5.73% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Multiple Sclerosis Therapeutic Market Size 2024 |
USD 27.4 billion |
| Multiple Sclerosis Therapeutic Market, CAGR |
5.73% |
| Multiple Sclerosis Therapeutic Market Size 2032 |
USD 42.8 billion |
The Multiple Sclerosis Therapeutic Market is driven by rising disease prevalence, growing diagnosis rates, and strong demand for advanced therapies. It benefits from innovation in biologics, oral formulations, and disease-modifying treatments that improve efficacy and patient adherence. Supportive regulatory frameworks and reimbursement policies further encourage adoption across developed regions. Trends highlight a shift toward personalized medicine, digital health integration, and AI-driven monitoring tools that optimize treatment outcomes. Increasing research in remyelination and neuroprotection also shapes future opportunities. It reflects a dynamic market focused on innovation, patient-centric care, and expanding global access to effective therapeutic solutions.
The Multiple Sclerosis Therapeutic Market shows strong presence in North America and Europe due to advanced healthcare systems, high diagnosis rates, and wide access to innovative therapies, while Asia-Pacific emerges with rapid growth supported by improving infrastructure and rising awareness. Latin America and the Middle East & Africa contribute smaller shares but offer expansion potential. Key players shaping the market include AbbVie, Biogen, Novartis, Roche, Bayer, Merck, Bristol-Myers Squibb, Johnson & Johnson, Amgen, and Acorda Therapeutics.
Market Insights
- The Multiple Sclerosis Therapeutic Market was valued at USD 27.4 billion in 2024 and is projected to reach USD 42.8 billion by 2032, growing at a CAGR of 5.73%.
- Rising prevalence of multiple sclerosis, growing diagnosis rates, and demand for advanced therapies drive steady growth.
- Innovation in biologics, oral formulations, and disease-modifying treatments strengthens efficacy and improves patient adherence.
- Market competition remains high with strong presence of AbbVie, Biogen, Novartis, Roche, Bayer, Merck, Bristol-Myers Squibb, Johnson & Johnson, Amgen, and Acorda Therapeutics.
- High treatment costs and limited access to advanced therapies in emerging regions act as restraints.
- North America and Europe lead the market, while Asia-Pacific shows the fastest growth with improving infrastructure and awareness.
- The market moves toward personalized medicine, digital health integration, AI-driven monitoring, and research in remyelination and neuroprotection for future opportunities.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Rising Prevalence of Multiple Sclerosis and Expanding Patient Pool
The Multiple Sclerosis Therapeutic Market benefits from the growing global prevalence of the disease. Rising diagnosis rates and better access to neurological care contribute to a larger patient base. The demand for effective therapies strengthens with early detection and improved awareness programs. Governments and healthcare providers also support initiatives that increase screening and specialist access. Expanding patient populations drive consistent uptake of disease-modifying therapies. It creates steady growth opportunities for both established pharmaceutical firms and new entrants.
- For instance, National Multiple Sclerosis Society invested $19 million to launch 40 new multi-year research awards in 2022 to drive progress toward finding new treatments and eventually a cure for multiple sclerosis.
Strong Pipeline of Innovative Therapies Driving Market Expansion
The Multiple Sclerosis Therapeutic Market advances through a robust pipeline of innovative treatment options. Biologics and monoclonal antibodies dominate late-stage trials, offering improved efficacy. The approval of new oral therapies provides patients with alternatives to injectable treatments. Breakthroughs in remyelination and neuroprotection research strengthen long-term market potential. Companies invest heavily in R&D collaborations to accelerate drug discovery. It ensures a steady stream of novel therapies designed to address unmet clinical needs.
- For instance, in Phase II studies, Roche’s BTK inhibitor fenebrutinib achieved near-complete suppression of disease signs and halted disability progression for up to 48 weeks in people with relapsing MS.
Impact of Regulatory Support and Favorable Reimbursement Policies
The Multiple Sclerosis Therapeutic Market gains momentum from supportive regulatory frameworks worldwide. Fast-track approvals and orphan drug designations accelerate the entry of new therapies. National healthcare systems increasingly reimburse disease-modifying treatments, reducing patient burden. Favorable insurance coverage promotes greater adoption of high-cost therapies. Governments emphasize long-term disease management, strengthening funding allocations. It improves accessibility and encourages pharmaceutical companies to launch advanced therapies across diverse markets.
Growing Role of Personalized Medicine and Advanced Treatment Approaches
The Multiple Sclerosis Therapeutic Market shifts toward personalized medicine approaches tailored to individual patient profiles. Biomarker research and genetic insights enable targeted treatment strategies. Advanced imaging tools assist in monitoring disease progression more effectively. The integration of AI-driven analytics helps clinicians optimize therapeutic outcomes. Patients benefit from customized dosing and reduced side effects. It positions precision medicine as a central driver of treatment innovation in the years ahead.
Market Trends
Increasing Adoption of Oral Therapies Transforming Treatment Landscape
The Multiple Sclerosis Therapeutic Market shows a strong shift toward oral formulations over injectables. Patients prefer oral drugs due to convenience and reduced discomfort. Pharmaceutical companies respond by expanding portfolios with high-efficacy oral therapies. The introduction of sphingosine-1-phosphate receptor modulators drives adoption in relapsing forms. Physicians increasingly prescribe these treatments for better adherence and quality of life. It strengthens the transition from traditional injectable regimens toward patient-friendly solutions.
- For instance, Acorda Therapeutics’ oral therapy Ampyra (dalfampridine) was tested in two Phase III trials collectively enrolling 540 MS patients with walking impairment. The regimen consisted of 10 mg sustained-release tablets taken twice daily, aimed at improving walking speed in MS patients.
Expanding Focus on Monoclonal Antibodies and Advanced Biologics
The Multiple Sclerosis Therapeutic Market highlights rapid expansion in monoclonal antibody usage. Biologics demonstrate superior efficacy in reducing relapse rates and delaying progression. Ocrelizumab and natalizumab represent key benchmarks in clinical performance. Companies invest in next-generation biologics targeting novel pathways. High clinical success rates reinforce physician confidence in these therapies. It accelerates the replacement of older disease-modifying drugs with advanced biologic alternatives.
- For instance, Bayer introduced nabiximols (Sativex), an oromucosal spray, as an add-on therapy for MS-related spasticity. In a 2011 Phase III study (Novotna et al.), approximately 47% of participants were categorized as initial responders based on showing at least a 20% improvement in spasticity on a 0–10 Numerical Rating Scale (NRS). In the double-blind, randomized phase that followed, responders who continued with nabiximols showed a statistically significant improvement in spasticity compared to those who switched to placebo.
Rising Emphasis on Neuroprotection and Remyelination Therapies
The Multiple Sclerosis Therapeutic Market is witnessing greater attention toward remyelination research. Neuroprotective drugs are under investigation to slow irreversible disability. Clinical studies explore agents that repair damaged myelin and restore function. Leading pharmaceutical firms collaborate with academic institutions to advance discovery programs. Patients and healthcare providers express interest in treatments addressing disease progression directly. It signals a long-term shift from symptom management toward regenerative approaches.
Integration of Digital Health and Personalized Treatment Approaches
The Multiple Sclerosis Therapeutic Market embraces digital health tools to enhance care delivery. Wearable devices and mobile platforms track symptoms in real time. AI-driven solutions support early relapse detection and personalized dosing. Telemedicine services expand patient access to specialists worldwide. Data-driven decision-making improves adherence and clinical outcomes. It reinforces the trend toward personalized treatment strategies tailored to individual disease profiles.
Market Challenges Analysis
High Treatment Costs and Limited Accessibility to Advanced Therapies
The Multiple Sclerosis Therapeutic Market faces significant barriers due to the high cost of advanced therapies. Disease-modifying drugs and biologics often remain unaffordable for many patients without strong insurance coverage. Reimbursement gaps across regions restrict access, especially in low- and middle-income countries. The financial burden discourages consistent treatment adherence, leading to compromised clinical outcomes. High expenses also place pressure on healthcare systems, limiting broader adoption of next-generation therapies. It creates disparities in patient care and slows down uniform market growth across global regions.
Complex Disease Management and Safety Concerns with Long-Term Therapy
The Multiple Sclerosis Therapeutic Market encounters challenges related to the complex nature of disease management. Patients often require lifelong treatment, yet long-term use of biologics raises safety concerns. Risks include infections, infusion-related reactions, and potential organ complications. Monitoring disease progression and therapy effectiveness demands advanced diagnostic tools, which are not always accessible. Limited biomarkers for predicting treatment response complicate therapy selection for physicians. It underscores the need for safer, targeted options that can balance efficacy with minimal adverse effects.
Market Opportunities
Expansion of Novel Therapeutics and Pipeline Advancements
The Multiple Sclerosis Therapeutic Market presents strong opportunities through innovative drug development. Advances in monoclonal antibodies, oral therapies, and regenerative agents expand treatment diversity. Emerging therapies focused on remyelination and neuroprotection aim to address unmet needs. Pharmaceutical companies benefit from robust pipelines supported by global research collaborations. Breakthrough approvals create pathways for faster adoption in both developed and emerging markets. It enhances prospects for therapies that can slow progression and improve long-term patient outcomes.
Growing Role of Digital Health and Personalized Medicine
The Multiple Sclerosis Therapeutic Market gains opportunities through digital health integration and tailored care models. AI-powered platforms support physicians with predictive analytics for relapse management. Remote monitoring tools and telehealth expand specialist access in underserved regions. Personalized medicine driven by biomarker identification improves treatment precision. Patients increasingly prefer customized regimens that balance efficacy with reduced side effects. It creates a long-term growth pathway by combining advanced science with patient-centered healthcare solutions.
Market Segmentation Analysis:
By Drug Class
The Multiple Sclerosis Therapeutic Market is segmented into immunosuppressants, immunostimulants, and immunomodulators. Immunomodulators hold a dominant share due to their effectiveness in reducing relapse frequency and slowing disease progression. Drugs such as interferon-beta and glatiramer acetate remain widely prescribed for relapsing-remitting multiple sclerosis. Immunosuppressants, including monoclonal antibodies, have gained traction for their high efficacy in controlling aggressive forms of the disease. Immunostimulants play a supportive role, though their use is less extensive compared to the other classes. It reflects the growing preference for therapies that balance safety with proven clinical benefits.
- For instance, AbbVie’s and Biogen’s once-monthly injectable daclizumab (Zinbryta) was initially supported for use in relapsing multiple sclerosis (MS) by positive results from the DECIDE Phase III trial, which involved about 1,841 patients. The study compared daclizumab administered every four weeks against interferon beta-1a, and it showed superior efficacy in reducing relapses and brain lesions.
By Route of Administration
The Multiple Sclerosis Therapeutic Market segments by administration into oral and injectable forms, which include intramuscular and intravenous delivery. Oral drugs show rapid adoption due to patient convenience and higher adherence rates. Products like fingolimod and siponimod highlight the shift toward easy-to-use formulations. Intramuscular injections maintain relevance but face declining usage compared to other modes. Intravenous therapies such as ocrelizumab continue to expand, particularly in hospitals, due to strong efficacy profiles. It highlights the balance between convenience-driven oral therapies and high-performance injectables in clinical practice.
By Distribution Channel
The Multiple Sclerosis Therapeutic Market is distributed through hospital, retail, and online pharmacies. Hospitals dominate due to specialist-led treatment initiation and monitoring requirements for advanced therapies. Retail pharmacies play an important role in recurring drug supply for patients on long-term regimens. Online pharmacies gain traction through growing digital adoption and demand for home delivery. Expansion of e-pharmacy platforms supports greater accessibility for patients in remote areas. It underlines a diversified channel landscape where traditional and digital pathways both contribute to market reach.
Segments:
Based on Drug Class:
- Immunosuppressants
- Immunostimulants
- Immunomodulators
Based on Route of Administration:
- Oral
- Injectable
- Intramuscular
Based on Distribution Channel:
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America holds the largest share of the Multiple Sclerosis Therapeutic Market, around 40%. The United States leads with high diagnosis rates, strong healthcare systems, and wide access to advanced treatments. Canada also supports growth with public healthcare coverage for multiple sclerosis therapies. Drug innovation, high adoption of biologics, and supportive regulations strengthen the region’s leadership. Companies benefit from early approvals and strong clinical research networks. It continues to attract investment due to its large patient base and advanced infrastructure.
Europe
Europe accounts for about 30% of the market. Countries like Germany, the UK, and France remain leaders in diagnosis and therapy adoption. The region benefits from structured healthcare policies and reimbursement programs that support patients with high-cost treatments. Research centers in Europe play a major role in clinical trials and pipeline development. Biologics and oral therapies see strong demand as patients seek more effective options. It sustains steady growth through advanced healthcare networks and government support.
Asia-Pacific
Asia-Pacific represents nearly 15% of the Multiple Sclerosis Therapeutic Market. Countries such as Japan, China, India, and Australia are key growth drivers. Rising awareness, better diagnosis, and expanding healthcare facilities increase demand for new therapies. Governments invest in neurology centers and encourage partnerships with pharmaceutical companies. Oral therapies and affordable treatment options find higher acceptance in this region. It shows the fastest growth rate, supported by a large patient pool and improving healthcare access.
Latin America
Latin America holds around 8% of the market. Brazil leads due to its larger healthcare system and government support for neurological care. Mexico and Argentina also contribute through expanding diagnostic capabilities and therapy adoption. Limited access and affordability challenges restrict broader growth in some areas. Pharmaceutical companies are exploring partnerships to expand their presence. It shows steady progress, with more patients expected to access advanced treatments in the coming years.
Middle East & Africa
The Middle East & Africa contributes about 7% to the Multiple Sclerosis Therapeutic Market. Countries such as Saudi Arabia and the UAE invest in improving rare disease treatment infrastructure. Awareness campaigns and new healthcare initiatives support growth in the region. Access remains uneven, with rural areas facing challenges in diagnosis and therapy availability. Multinational companies are entering the market through collaborations with local healthcare providers. It represents a smaller share but offers opportunities for long-term expansion.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
Competitive Analysis
The Multiple Sclerosis Therapeutic Market players include AbbVie Inc., Amgen Inc., Acorda Therapeutics, Inc., Biogen Inc., Bayer AG, Bristol-Myers Squibb Company, F. Hoffmann-La Roche Ltd, Johnson & Johnson, Merck & Co., Inc., and Novartis AG. The Multiple Sclerosis Therapeutic Market remains highly competitive, driven by continuous innovation, strong pipelines, and the introduction of advanced biologics and oral therapies. Companies focus on expanding treatment options for both relapsing and progressive forms of the disease while addressing unmet clinical needs. Strategic partnerships, research collaborations, and large-scale clinical trials support the launch of next-generation therapies with improved safety and efficacy profiles. Market players emphasize digital health integration, patient-centric treatment models, and personalized medicine to strengthen adoption. The competitive landscape is further shaped by regulatory approvals, pricing strategies, and regional expansion, ensuring steady growth and evolving opportunities for novel therapies worldwide.
Recent Developments
- In June 2025, CivicaScript, LLC, declared the availability of dimethyl fumarate delayed-relapse capsules which is a low-cost medication indicated for treating relapsing forms of multiple sclerosis (MS) in adults.
- In March 2025, Clario, a leader in providing endpoint data solutions for the clinical trial industry, acquired NeuroRx, a leading imaging analysis company with expertise in multiple sclerosis.
- In March 2024, Juvisé Pharmaceuticals, a French pharmaceutical company, acquired global commercial rights (excluding the United States and Canada) to Ponvory (ponesimod), indicated for the treatment of adults with active forms of relapsing multiple sclerosis (RMS), from Actelion Pharmaceuticals Ltd, a Johnson & Johnson Company (Johnson & Johnson).
- In February 2024, F. Hoffmann La-Roche Ltd announced the launch of its breakthrough drug, Ocrevus (Ocrelizumab), for treating multiple sclerosis (MS), expanding its neurology portfolio to address the unmet needs of patients in India.
Report Coverage
The research report offers an in-depth analysis based on Drug Class, Route of Administration, Distribution Channel and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will see rising adoption of oral therapies due to patient convenience.
- Biologics and monoclonal antibodies will continue to gain strong demand.
- Personalized medicine will expand with biomarker-based treatment strategies.
- Digital health tools will improve monitoring and treatment adherence.
- Research in remyelination and neuroprotection will shape next-generation therapies.
- Regulatory support will accelerate approvals of advanced disease-modifying drugs.
- Growing awareness programs will increase early diagnosis and treatment uptake.
- Emerging markets will expand access through better healthcare infrastructure.
- Strategic collaborations will drive faster innovation and pipeline development.
- The market will focus on safer therapies with improved long-term outcomes.




