Market Overview
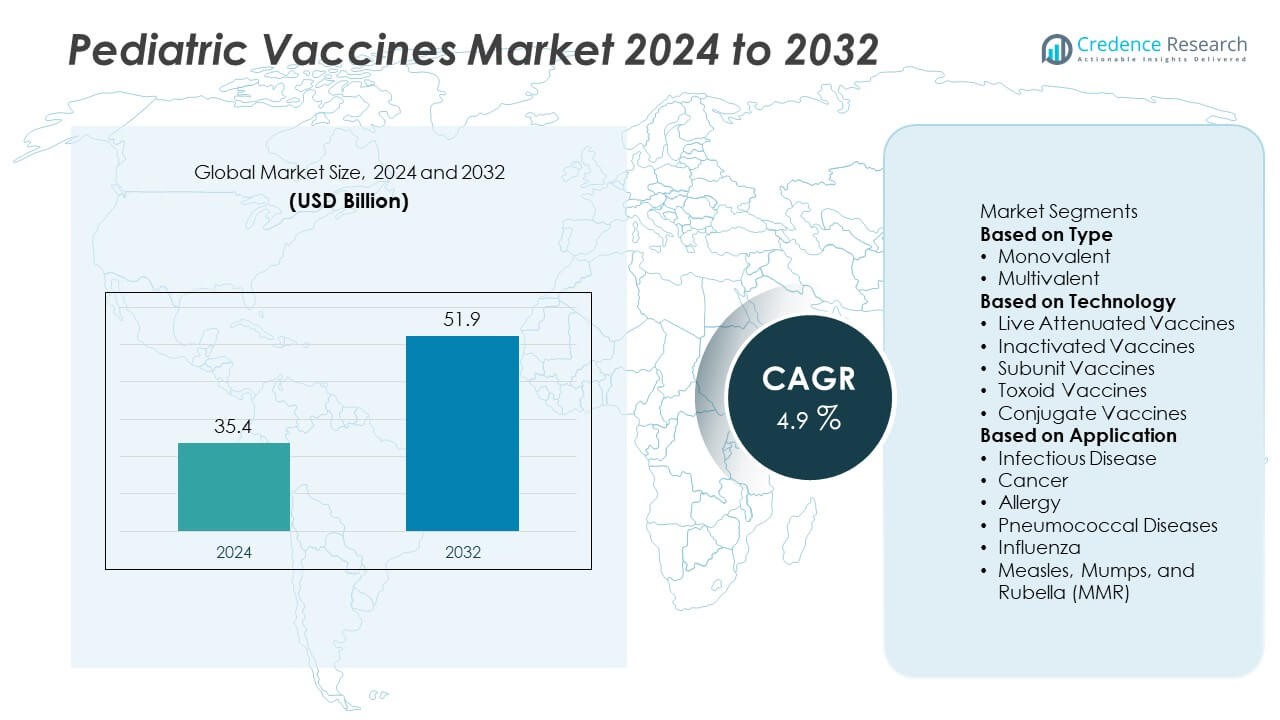
The Pediatric Vaccines Market was valued at USD 35.4 billion in 2024 and is projected to reach USD 51.9 billion by 2032, growing at a CAGR of 4.9% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Pediatric Vaccines Market Size 2024 |
USD 35.4 Billion |
| Pediatric Vaccines Market, CAGR |
4.9% |
| Pediatric Vaccines Market Size 2032 |
USD 51.9 Billion |
The Pediatric Vaccines Market is driven by rising government immunization programs, increasing parental awareness of preventive healthcare, and continuous technological advancements that enhance vaccine safety and effectiveness. Strong demand for combination vaccines that simplify schedules and improve compliance supports market expansion.
The Pediatric Vaccines Market demonstrates significant geographical diversity, with strong adoption across North America and Europe supported by advanced healthcare infrastructure, structured immunization programs, and ongoing research initiatives. Asia-Pacific emerges as the fastest-growing region, fueled by large pediatric populations, rising government investments, and expanding vaccine accessibility in countries such as India and China. Latin America and the Middle East & Africa show steady growth with increasing government campaigns and international support to improve vaccination rates despite challenges in rural coverage. Key players shaping the competitive landscape include Pfizer, Inc., recognized for its global vaccine portfolio and innovation in pediatric immunization, Sanofi, which remains a leader in both routine and combination vaccines, and the Serum Institute of India Pvt. Ltd., known for its large-scale vaccine production and global distribution. GSK plc also holds a strong presence with advanced research capabilities and a comprehensive pediatric vaccine portfolio.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Pediatric Vaccines Market was valued at USD 35.4 billion in 2024 and is projected to reach USD 51.9 billion by 2032, growing at a CAGR of 4.9% during the forecast period.
- Rising government-backed immunization programs and greater parental awareness about preventive healthcare are key drivers ensuring consistent demand for pediatric vaccines.
- Combination vaccines, next-generation platforms such as mRNA, and increasing investment in cold chain infrastructure are shaping market trends and improving global accessibility.
- Competitive dynamics are defined by leading players such as Pfizer, Sanofi, GSK plc, and the Serum Institute of India Pvt. Ltd., along with regional players expanding reach through partnerships and innovation.
- High development and distribution costs, coupled with vaccine hesitancy in certain populations, remain significant restraints that limit wider adoption and accessibility in some regions.
- North America and Europe lead the market with robust healthcare systems and structured immunization programs, while Asia-Pacific demonstrates the fastest growth due to large pediatric populations and expanding healthcare investments.
- Latin America and the Middle East & Africa show steady improvement, supported by national vaccination campaigns and international initiatives aimed at addressing infrastructure gaps and expanding
Market Drivers
Rising Global Focus on Childhood Immunization Programs
Governments and health organizations continue to prioritize childhood immunization as a critical public health measure. The Pediatric Vaccines Market benefits from national and international programs aimed at expanding vaccine coverage and reducing preventable diseases. Large-scale initiatives supported by the World Health Organization and UNICEF improve access in low- and middle-income countries. Public health campaigns encourage early vaccination to lower the burden of infectious diseases in young populations. Strong policy support ensures steady demand and creates opportunities for new vaccine introductions. It reflects a growing commitment to universal immunization standards.
- For instance, Pfizer committed to supplying UNICEF with 260 million doses of Prevnar 13 pneumococcal vaccine through supply agreements-on top of 480 million previously committed doses-helping extend protection in the world’s poorest countries.
Growing Awareness Among Parents About Preventive Healthcare
Parents increasingly recognize the importance of timely vaccination to safeguard children from life-threatening infections. The Pediatric Vaccines Market gains traction from rising awareness about disease prevention through reliable immunization. Greater access to digital platforms and health education programs helps parents make informed choices. Expanding healthcare outreach in urban and rural areas strengthens knowledge about the benefits of vaccination. Trust in pediatricians and healthcare workers supports vaccine uptake and compliance. It reinforces the role of education and awareness in sustaining long-term market growth.
- For instance, GlaxoSmithKline’s Rotarix vaccine surpassed 100 million doses administered globally by 2011, not 2022. This milestone, which protected an estimated 50 million children, helped reduce hospitalizations for severe rotavirus gastroenteritis.
Technological Advancements and Innovative Vaccine Development
Continuous innovation in biotechnology drives the availability of safer, more effective pediatric vaccines. The Pediatric Vaccines Market grows with the introduction of combination vaccines that reduce the number of injections for children. Advances in cold chain logistics and delivery methods improve efficiency and reliability. Pharmaceutical companies invest heavily in research pipelines to address unmet needs in pediatric immunization. Expedited regulatory approvals for breakthrough vaccines accelerate product availability in global markets. It strengthens confidence among healthcare providers and parents in adopting new immunization solutions.
Rising Prevalence of Infectious Diseases in Children
The increasing incidence of infectious diseases among children heightens the demand for effective immunization strategies. The Pediatric Vaccines Market experiences strong momentum from the urgent need to address recurring outbreaks. Seasonal flu, respiratory infections, and emerging viral threats continue to pressure healthcare systems. Growing urbanization and global travel increase exposure risks for young populations. Governments and medical institutions emphasize preventive vaccination to limit disease transmission. It underscores the vital role of pediatric vaccines in protecting community health and ensuring long-term resilience.
Market Trends
Increasing Adoption of Combination Vaccines for Better Compliance
Healthcare providers continue to prefer combination vaccines that protect against multiple diseases with fewer injections. The Pediatric Vaccines Market benefits from this trend by improving compliance among children and reducing clinic visits. Parents favor such options because they minimize discomfort and simplify vaccination schedules. Pharmaceutical companies respond with new formulations designed to enhance protection and convenience. Regulatory agencies also encourage the development of combination vaccines to improve coverage rates. It positions combination vaccines as a key growth driver in future immunization strategies.
- For instance, in two U.S.-based clinical studies evaluating VAXELIS (a six-in-one DTaP-IPV-Hib-HepB vaccine), research involved 986 infants in one group and 2,406 in another, with results demonstrating non-inferior immune responses compared to traditional separate vaccines, illustrating scalable effectiveness of combination formulations.
Expansion of Public–Private Partnerships to Improve Access
Collaborations between governments, non-profit organizations, and private companies are strengthening pediatric immunization efforts. The Pediatric Vaccines Market sees significant support from these partnerships that aim to expand affordability and access. Joint initiatives provide funding, logistics, and distribution in underserved regions. Partnerships also enable better training for healthcare workers to ensure efficient vaccine delivery. Industry players gain from wider outreach while public health systems achieve stronger disease control. It highlights the role of cooperation in improving immunization infrastructure worldwide.
- For instance, since its inception in 2000, Gavi has supported 637 vaccine introductions and immunization campaigns targeting 16 infectious diseases in lower-income countries, demonstrating substantial programmatic breadth and reach.
Rising Investment in Cold Chain and Supply Chain Innovations
Reliable storage and transportation remain critical for preserving vaccine effectiveness. The Pediatric Vaccines Market is witnessing steady investment in cold chain technologies that ensure safe delivery in both developed and developing regions. Advanced monitoring systems help track temperature stability and reduce wastage. Companies are adopting digital tools to streamline logistics and enhance distribution efficiency. Governments encourage infrastructure upgrades to prevent supply interruptions during crises. It demonstrates how supply chain resilience is becoming central to pediatric vaccine availability.
Growing Preference for Novel Platforms and Next-Generation Vaccines
Innovation in vaccine platforms is reshaping the landscape of pediatric immunization. The Pediatric Vaccines Market is moving toward next-generation solutions such as mRNA and viral vector vaccines. These platforms offer faster development timelines and strong immune responses against evolving pathogens. Pharmaceutical companies invest heavily in research to adapt these technologies for pediatric use. Healthcare providers and regulators show growing acceptance of such advanced vaccines. It signals a shift toward more adaptable and efficient immunization methods for children.
Market Challenges Analysis
High Costs of Vaccine Development and Distribution
The Pediatric Vaccines Market faces persistent challenges due to the high costs linked to research, development, and large-scale production. Developing safe and effective pediatric vaccines requires significant investment in clinical trials, testing, and regulatory approvals. Small and medium-sized manufacturers struggle to compete with larger players because of limited financial resources. Distribution costs also remain high, especially in regions with weak healthcare infrastructure. Cold chain requirements further add to the overall expense, making it difficult to ensure affordability in low-income areas. It creates a financial barrier that can restrict vaccine accessibility for vulnerable populations.
Vaccine Hesitancy and Uneven Global Coverage
Growing vaccine hesitancy among parents presents a major obstacle to wider adoption of pediatric vaccines. The Pediatric Vaccines Market suffers when misinformation, cultural beliefs, and distrust in healthcare systems influence parental decisions. Uneven vaccine coverage remains evident between urban and rural areas, and between developed and developing countries. Limited awareness in certain communities hinders timely immunization schedules. Healthcare providers face difficulties in countering misinformation while ensuring compliance with vaccination programs. It emphasizes the need for targeted education and trust-building initiatives to overcome hesitancy and achieve broader immunization coverage.
Market Opportunities
Expansion into Emerging Markets with Strong Growth Potential
The Pediatric Vaccines Market holds significant opportunities in emerging economies where demand for immunization is steadily rising. Governments in these regions invest heavily in healthcare infrastructure to improve vaccination access. Rising birth rates create a large target population, increasing the need for affordable and effective pediatric vaccines. International health organizations also support immunization drives, enabling wider distribution and coverage. Pharmaceutical companies can expand their presence by forming partnerships with local players to strengthen supply networks. It underscores the opportunity for manufacturers to capture untapped markets and build long-term growth.
Advancement of Next-Generation Vaccines and Delivery Methods
Rapid innovation in biotechnology creates strong potential for developing next-generation pediatric vaccines. The Pediatric Vaccines Market benefits from research into novel platforms such as mRNA, DNA-based, and nanoparticle vaccines. These technologies promise enhanced safety, quicker production, and adaptability to evolving infectious threats. Alternative delivery methods, including needle-free systems, can improve compliance and reduce fear among children. Investors and healthcare providers show growing interest in supporting these innovations for future adoption. It positions the industry to capitalize on scientific progress while addressing unmet needs in pediatric immunization.
Market Segmentation Analysis:
By Type
The Pediatric Vaccines Market is divided into monovalent and multivalent vaccines, each addressing different healthcare needs. Monovalent vaccines remain essential in targeting individual diseases where focused immunization is critical, especially in regions with high prevalence of specific infections. Multivalent vaccines are gaining stronger acceptance due to their ability to provide protection against multiple diseases with fewer doses. Parents and healthcare providers increasingly prefer these vaccines for convenience and reduced clinical visits. Pharmaceutical companies continue to invest in this category, aiming to expand access to combination solutions. It reflects how type segmentation supports both traditional and innovative immunization strategies.
- For instance, the Pfizer–BioNTech BNT162b2 Phase 2–3 trial administered 3‑microgram doses to children aged 6 months to under 2 years (1,178 recipients) and 2 to 4 years (1,835 recipients) across three doses—and achieved immunobridging success compared to adults.
By Technology
The market is segmented into live attenuated vaccines, inactivated vaccines, subunit vaccines, toxoid vaccines, conjugate vaccines, and others. Live attenuated vaccines demonstrate strong efficacy and long-lasting immunity, making them vital for common pediatric diseases. Inactivated vaccines maintain steady demand where safety and stability are critical considerations. Subunit and conjugate vaccines are gaining traction for their targeted action and lower risk profiles, especially for young children. Toxoid vaccines continue to hold importance in preventing bacterial infections such as diphtheria and tetanus. It underscores the role of diverse technologies in addressing global pediatric health challenges.
- For instance, during one study, nearly 396,173 children received live attenuated influenza vaccine, totaling 590,018 doses administered in the study period.
By Application
The market covers infectious diseases, cancer, allergy, pneumococcal diseases, influenza, measles, mumps and rubella (MMR), and other conditions. Infectious diseases dominate the segment due to global vaccination programs that aim to reduce child mortality. Vaccines for pneumococcal diseases and influenza sustain consistent demand because of recurring seasonal threats and public health priorities. MMR vaccines remain standard in routine immunization schedules worldwide, supporting long-term disease control. Cancer and allergy vaccines represent emerging opportunities supported by advances in biotechnology and research funding. It highlights the breadth of applications that drive market growth while addressing both established and emerging pediatric health concerns.
Segments:
Based on Type
Based on Technology
- Live Attenuated Vaccines
- Inactivated Vaccines
- Subunit Vaccines
- Toxoid Vaccines
- Conjugate Vaccines
Based on Application
- Infectious Disease
- Cancer
- Allergy
- Pneumococcal Diseases
- Influenza
- Measles, Mumps, and Rubella (MMR)
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America accounts for 32% of the global Pediatric Vaccines Market share. The region benefits from advanced healthcare systems, favorable government initiatives, and a strong pharmaceutical presence. The United States leads with high vaccination coverage rates and consistent inclusion of new vaccines in routine schedules. Regulatory support from the FDA and CDC strengthens public confidence in immunization programs. Canada maintains a steady position with government-funded campaigns and increasing focus on preventive healthcare. The region shows sustained momentum with steady adoption of advanced vaccine technologies and combination formulations.
Europe
Europe contributes 28% of the global Pediatric Vaccines Market share. Leading economies such as Germany, France, and the United Kingdom drive growth through well-structured immunization programs and strong pediatric research. Coordinated efforts by the ECDC promote harmonized vaccination strategies across member states. Government funding and insurance support ensure accessibility and affordability across populations. Europe continues to see rising demand for multivalent vaccines and next-generation technologies to address new infectious threats, reflecting its established infrastructure and high adoption rates.
Asia-Pacific
Asia-Pacific holds 25% of the global Pediatric Vaccines Market share and demonstrates the fastest growth. Rising birth rates, expanding healthcare infrastructure, and greater government investment in immunization drive momentum. China and India account for the largest demand due to their pediatric population size and national vaccination programs. Supportive policies from Gavi and growing awareness among parents further strengthen regional coverage. Japan, South Korea, and Australia add to demand with advanced vaccine adoption and strong research activity. Asia-Pacific also emerges as a hub for large-scale vaccine manufacturing.
Latin America
Latin America contributes 9% of the global Pediatric Vaccines Market share. Brazil and Mexico lead adoption through national immunization campaigns and improving healthcare access. Public-private partnerships enhance supply chain efficiency and strengthen distribution systems. Challenges remain in rural areas due to uneven healthcare infrastructure. Nonetheless, international collaborations support expanding coverage, making Latin America an opportunity-rich region for global players targeting affordable vaccine distribution.
Middle East and Africa
The Middle East and Africa account for 6% of the global Pediatric Vaccines Market share. Higher adoption is visible in GCC countries like Saudi Arabia and the UAE, where healthcare systems are well developed. In contrast, many sub-Saharan African regions face limited infrastructure and high disease prevalence, slowing coverage. Support from WHO, UNICEF, and Gavi remains vital in expanding vaccination in underserved areas. With increasing international aid and government-backed programs, this region holds strategic importance for future vaccine accessibility and growth potential.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- AstraZeneca
- Zydus Group
- BIO-MED
- SINOVAC
- Pfizer, Inc.
- Serum Institute of India Pvt. Ltd.
- Panacea Biotec
- GSK plc
- Indian Immunologicals Ltd.
- Sanofi
Competitive Analysis
The competitive landscape of the Pediatric Vaccines Market is shaped by leading players including Pfizer, Inc., Sanofi, GSK plc, Serum Institute of India Pvt. Ltd., AstraZeneca, Zydus Group, Indian Immunologicals Ltd., Panacea Biotec, SINOVAC, and BIO-MED. These companies drive innovation, large-scale production, and distribution to meet global immunization needs. Multinational corporations such as Pfizer, Sanofi, and GSK leverage strong research pipelines, advanced technologies, and global networks to maintain dominance in developed regions. Emerging players, particularly Serum Institute of India and Panacea Biotec, focus on high-volume manufacturing and affordable vaccine supply for developing countries. Strategic collaborations, partnerships with governments, and support from organizations like Gavi further enhance market reach. Continuous investment in next-generation platforms, including mRNA and combination vaccines, positions key players to address evolving healthcare demands. Competitive intensity remains high as both established leaders and regional manufacturers strive to expand access, strengthen portfolios, and adapt to global immunization priorities.
Recent Developments
- In June 2025, Sanofi initiated early shipment of its RSV antibody product, Beyfortus, ahead of the 2025–2026 RSV season.
- In June 2025, SIIPL became the first manufacturer to submit a WHO prequalification dossier in the electronic CTD format.
- In May 2025, the FDA expanded approval of MenQuadfi (meningococcal ACWY vaccine) to include infants as young as 6 weeks.
- In April 2023, the U.S. FDA approved Pfizer’s PREVNAR 20 (PCV20) in April 2023 for the prevention of invasive pneumococcal disease (IPD) in individuals six weeks through 17 years of age.
Report Coverage
The research report offers an in-depth analysis based on Type, Technology, Application and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will expand with rising government investment in universal immunization programs.
- Demand will increase for multivalent vaccines that reduce the number of doses for children.
- Biotechnology advancements will accelerate the development of safer and more effective pediatric vaccines.
- Emerging economies will provide strong growth opportunities due to large pediatric populations.
- Next-generation platforms such as mRNA and viral vector vaccines will gain wider adoption in pediatrics.
- Cold chain and supply chain improvements will strengthen vaccine distribution across underserved regions.
- Public–private partnerships will play a greater role in expanding access to affordable vaccines.
- Rising parental awareness and education will improve vaccine acceptance and compliance.
- Continuous innovation will lead to the introduction of new vaccines targeting both infectious and non-infectious diseases.
- Global collaborations will focus on equitable vaccine access, ensuring stronger market sustainability.




