Market Overview:
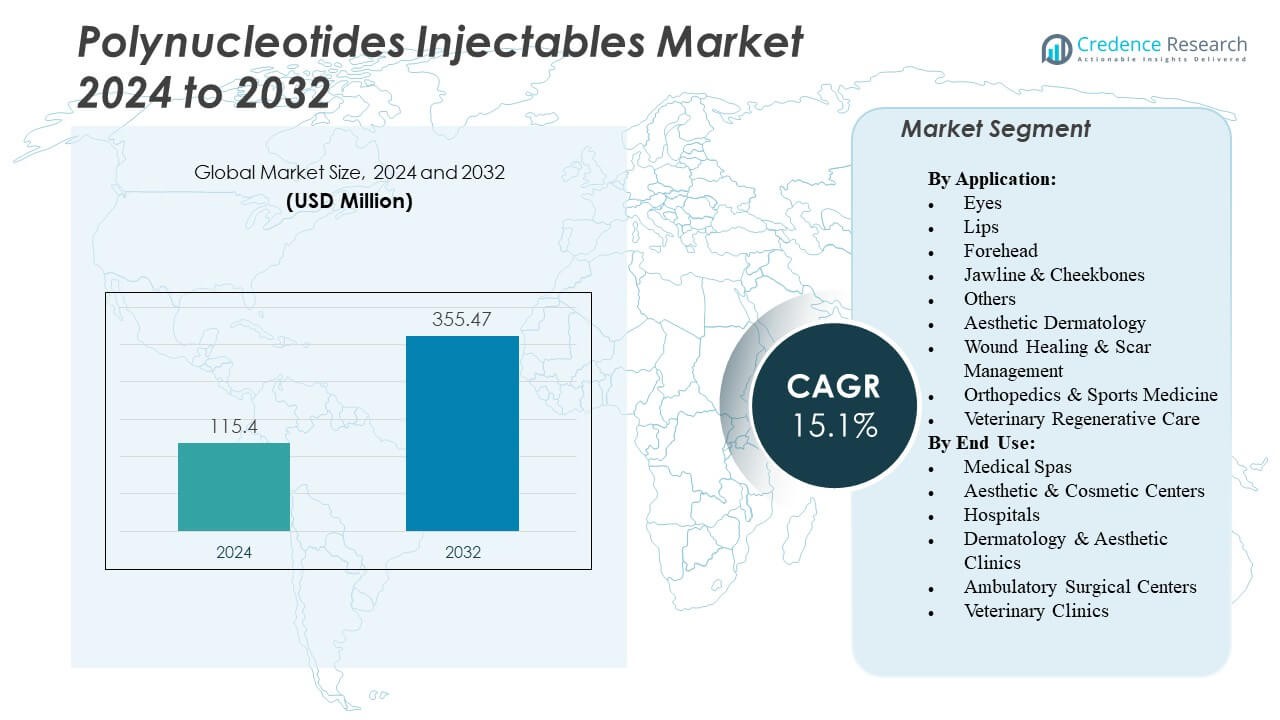
The Polynucleotides Injectables Market is projected to grow from USD 115.4 million in 2024 to an estimated USD 355.47 million by 2032, with a compound annual growth rate (CAGR) of 15.1% from 2024 to 2032.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Polynucleotides Injectables Market Size 2024 |
USD 115.4 Million |
| Polynucleotides Injectables Market, CAGR |
15.1% |
| Polynucleotides Injectables Market Size 2032 |
USD 355.47 Million |
Growing consumer interest in regenerative and biostimulatory aesthetic treatments drives this market. Dermatologists and clinics adopt these injectables for improving skin elasticity, texture, and hydration without surgical procedures. It supports collagen regeneration and long-term skin health, appealing to patients seeking natural results. Rising awareness of non-invasive rejuvenation options and technological improvements in product formulations further accelerate adoption across professional aesthetic practices.
Asia-Pacific leads the Polynucleotides Injectables Market with strong presence of advanced cosmetic clinics and high consumer demand. Countries such as South Korea, Japan, and China show strong adoption due to growing beauty consciousness and medical tourism. North America and Europe maintain steady growth through established clinical infrastructure and regulatory support for innovative injectables. Emerging regions in Latin America and the Middle East expand gradually, fueled by rising disposable incomes and greater awareness of advanced aesthetic solutions.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Polynucleotides Injectables Market is projected to grow from USD 115.4 million in 2024 to USD 355.47 million by 2032, registering a CAGR of 15.1% during the forecast period.
- Rising demand for regenerative and biostimulatory aesthetic procedures boosts market expansion across cosmetic and dermatology clinics.
- Growing consumer preference for natural, non-surgical rejuvenation enhances adoption among younger and aging populations alike.
- Technological advancements in DNA-based formulations improve product stability, bioavailability, and treatment outcomes.
- Limited clinical data availability and high regulatory compliance requirements restrain faster product approval and market entry.
- Asia-Pacific dominates the market, led by South Korea, Japan, and China, supported by advanced cosmetic facilities and aesthetic awareness.
- North America and Europe maintain steady growth, while Latin America and the Middle East emerge as expanding markets driven by growing disposable incomes.
Market Drivers
Growing Demand for Regenerative Aesthetic Procedures
The Polynucleotides Injectables Market experiences strong growth from the rising preference for regenerative and biostimulatory treatments in aesthetic medicine. Patients choose procedures that improve skin elasticity, hydration, and texture without invasive surgery. Dermatologists use these injectables to promote natural collagen synthesis and cellular repair. Demand grows across anti-aging clinics and cosmetic centers that emphasize long-term skin wellness. It benefits from the shift toward bioactive materials offering safer rejuvenation outcomes. Brands develop advanced formulations with DNA-based ingredients for enhanced results. Public awareness of natural regenerative therapies encourages adoption. Clinical validation further strengthens the treatment credibility among professionals.
- For instance, HTL Biotechnology produces high-purity polynucleotides (PDRN-PN) derived from sustainably sourced wild Alaskan salmon DNA for injectable and regenerative skin applications. Commercial PDRN typically exhibits a molecular weight peak near 132 kDa, within a broader 80–200 kDa range, supporting effective cellular repair and collagen stimulation in aesthetic dermatology.
Rising Adoption Across Dermatology and Aesthetic Clinics
Expanding use of polynucleotide injectables in dermatology and cosmetic practices drives market expansion. Dermatologists prefer these solutions for treating fine lines, scars, and skin laxity with minimal downtime. Clinics integrate them into advanced skin restoration protocols for consistent outcomes. It supports procedures combining fillers, PRP, and light therapies. The professional segment invests in devices and training to maintain treatment precision. Growing awareness of safe biocompatible injectables enhances trust among users. Beauty centers partner with licensed suppliers to meet increasing demand. This integration of biorevitalization therapies enhances clinic competitiveness in high-end cosmetic markets.
- For instance, PharmaResearch offers Rejuran Healer polynucleotide products optimized for collagen stimulation in fine line treatments.
Technological Advancements and Product Innovation
Continuous R&D investments by manufacturers boost technological progress in the Polynucleotides Injectables Market. Formulators develop stable, high-purity DNA polymers that increase bioavailability and tissue regeneration speed. It enhances the skin’s repair mechanism through improved hydration and elasticity. Companies integrate polynucleotides with hyaluronic acid for synergistic effects. Patented molecular refinement processes improve consistency and safety profiles. Advancements in microinjection systems ensure precise application with minimal tissue trauma. Clinical trials validate their long-lasting results compared to traditional dermal fillers. Innovation strengthens the overall market competitiveness among biotech-driven aesthetic manufacturers.
Growing Awareness and Expanding Consumer Demographics
Widening consumer awareness regarding non-surgical rejuvenation supports industry expansion. Younger demographics adopt injectables for early-age skin maintenance and prevention of dermal damage. It gains attention from wellness influencers who highlight results and recovery advantages. Cosmetic professionals educate clients on the regenerative mechanism of polynucleotides. Expanding social media exposure accelerates acceptance among global audiences. Male aesthetic care also contributes to the rising demand base. Clinics tailor marketing campaigns to attract various skin-type groups. Broader accessibility and education drive stronger adoption in both premium and mid-tier aesthetic markets.
Market Trends
Integration with Complementary Skin Rejuvenation Therapies
The Polynucleotides Injectables Market shows integration with advanced skin treatments such as microneedling and energy-based therapies. Combining procedures enhances absorption and repair at the cellular level. Clinics develop hybrid rejuvenation protocols using polynucleotides for faster visible recovery. It enables practitioners to address multiple aging concerns in one session. Dermatologists report improved elasticity and tone when pairing injectables with collagen stimulation tools. Growth of multifunctional cosmetic solutions accelerates innovation across formulations. Technology convergence appeals to premium clients seeking personalized outcomes. These hybrid approaches reflect a major transformation in clinical aesthetic practice.
Shift Toward DNA-Based and Bioengineered Formulations
Ongoing product innovation focuses on DNA-derived materials with enhanced regenerative performance. Brands design synthetic or bioengineered polynucleotides for superior tissue integration. It allows deeper cellular repair and antioxidant protection within skin layers. Advanced biofermentation methods improve ingredient purity and reproducibility. New formulations extend shelf life and maintain stability under clinical storage conditions. Manufacturers pursue medical-grade certifications to ensure safety consistency. Rising trust in scientific biocosmetics drives the shift from synthetic fillers toward bioactive injectables. This transition reflects growing consumer interest in sustainable and science-backed skincare technologies.
- For instance, PN-HPT® (Polynucleotides Highly Purified Technology) intradermal priming reduced skin texture from 29.1 to 16.1 units after 6 weeks in nasolabial folds.
Expansion of Medical Aesthetic Tourism in Asia-Pacific
Asia-Pacific becomes a hub for aesthetic tourism, promoting strong procedural growth in South Korea, Thailand, and Japan. Clinics attract international patients seeking affordable, advanced skin treatments. It benefits from competitive costs and trained practitioners offering certified injectables. Medical tourism agencies market rejuvenation packages combining treatment and travel. Regional manufacturers supply cost-efficient formulations to clinics catering to foreign visitors. Government health authorities support international safety standards to maintain credibility. Expanding clinic networks enhance cross-border awareness of bio-revitalization therapies. The influx of patients strengthens the global presence of Asian aesthetic service providers.
- For instance, HA-PN complex fillers maintained volume ratios over 24 weeks in dorsal skin models, outperforming HA alone in 3D volumetric analysis.
Rising Digital Marketing and Consumer Education Efforts
Social media and digital campaigns play a vital role in building patient confidence in polynucleotide injectables. Influencers and medical professionals share real-treatment experiences through verified online platforms. It drives curiosity and higher clinic footfall among beauty-conscious users. Digital consultations help potential clients understand pre- and post-procedure benefits. Clinics leverage AI-based imaging tools to simulate outcomes during marketing. Educational content about molecular science builds transparency in treatment mechanisms. Online reputation management becomes essential for attracting international clients. Strong digital engagement sustains long-term awareness and consumer trust in the therapy.
Market Challenges Analysis
Regulatory Complexities and Product Approval Delays
The Polynucleotides Injectables Market faces regulatory barriers that slow product introductions. Varying national rules for bio-derived substances complicate approval procedures. Manufacturers must provide long-term safety data to achieve clinical validation. It increases development costs and delays commercialization. Compliance with cosmetic and medical device frameworks requires extensive documentation. Small firms struggle with certification due to limited resources. Stringent labeling norms also affect cross-border trade. This regulatory fragmentation limits swift expansion despite rising demand across major economies.
Limited Clinical Data and Professional Training Gaps
A shortage of peer-reviewed studies challenges broader clinical acceptance. Dermatologists demand more evidence regarding durability and biocompatibility outcomes. It creates hesitation among practitioners unfamiliar with product mechanisms. Training programs remain limited in emerging markets, affecting injection precision. Uneven practitioner expertise increases risks of underperformance in aesthetic outcomes. Manufacturers invest in educational seminars to raise procedural competence. Lack of standardized treatment protocols reduces predictability across clinics. Closing these gaps remains essential for ensuring consistent quality and consumer confidence.
Market Opportunities
Expanding Use in Regenerative and Post-Procedure Applications
Growing interest in regenerative medicine creates new avenues for market expansion. Clinicians use polynucleotides in wound healing and post-laser recovery therapies. It improves tissue hydration and cellular turnover for faster recovery. Hospitals and dermatology centers evaluate their use in scar reduction and skin restoration. Biopharma collaborations focus on enhancing molecular stability for broader medical applications. Integration with other active ingredients drives innovation in hybrid aesthetic formulations. This growing medical utility expands the market beyond cosmetic indications. Demand from clinical dermatology supports sustained business growth.
Emerging Markets and Technological Globalization
Emerging economies in Asia, Latin America, and Eastern Europe present strong potential for expansion. Local distributors collaborate with international brands to meet rising aesthetic demands. It benefits from expanding urban populations adopting premium beauty care. Government focus on healthcare modernization improves import channels for advanced injectables. New manufacturing facilities lower cost barriers and increase regional supply availability. Online education platforms help professionals in new markets gain procedural expertise. Growing affordability and awareness create a favorable environment for product adoption. This shift supports the globalization of regenerative aesthetic solutions.
Market Segmentation Analysis:
By Application
The Polynucleotides Injectables Market shows strong demand across facial rejuvenation zones, including eyes, lips, forehead, and jawline. It supports treatments focused on elasticity restoration and collagen renewal, particularly in areas prone to fine lines and sagging. Clinics use these injectables to enhance firmness and hydration in sensitive regions. Aesthetic dermatology remains a dominant segment due to consistent client preference for non-surgical rejuvenation. Wound healing and scar management gain traction with proven tissue regeneration outcomes. Orthopedics and sports medicine segments adopt polynucleotide solutions for soft tissue repair. Veterinary regenerative care also expands, highlighting cross-specialty applications beyond cosmetics. This broad scope strengthens market penetration across diverse therapeutic areas.
- For instance, polynucleotides with hyaluronic acid achieved complete healing in 60% of venous lower limb ulcers at 45 days with 67% average area reduction versus 22% and 34% for hyaluronic acid alone.
By End Use
Medical spas and aesthetic centers lead the Polynucleotides Injectables Market through high treatment frequency and consumer awareness. It benefits from personalized care models emphasizing natural and regenerative results. Dermatology and aesthetic clinics play a vital role in integrating injectable therapies into advanced skin repair programs. Hospitals extend use for post-surgical recovery and wound care. Ambulatory surgical centers adopt injectables within minimally invasive procedures, ensuring efficiency and reduced downtime. Veterinary clinics explore these solutions for animal tissue recovery and dermal restoration. Expanding practitioner expertise and accessibility across medical facilities sustain consistent growth across all end-use categories.
- For instance, clinical studies show that PDRN therapy enhances healing in diabetic foot ulcers by stimulating angiogenesis and tissue regeneration. It improves granulation and epithelialization without adverse effects, confirming its therapeutic role in chronic wound management.
Segmentation:
By Application:
- Eyes
- Lips
- Forehead
- Jawline & Cheekbones
- Others
- Aesthetic Dermatology
- Wound Healing & Scar Management
- Orthopedics & Sports Medicine
- Veterinary Regenerative Care
By End Use:
- Medical Spas
- Aesthetic & Cosmetic Centers
- Hospitals
- Dermatology & Aesthetic Clinics
- Ambulatory Surgical Centers
- Veterinary Clinics
By Region:
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, South Korea, India, Australia, Rest of Asia-Pacific)
- Latin America (Brazil, Argentina, Rest of Latin America)
- Middle East & Africa (UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa)
Regional Analysis:
The Polynucleotides Injectables Market shows a clear regional leadership by Asia-Pacific, which held roughly 46.5% share of the global market in 2024. The region benefits from high demand for skin-rejuvenation, growing medical aesthetics infrastructure, and rising consumer awareness around minimally invasive procedures. It attracts significant patient volume due to a combination of affordability and increasing aesthetic standards in countries such as South Korea, China, and Southeast Asia. North America follows, contributing a substantial portion of global revenues. Many advanced clinics, strong regulatory frameworks, and high consumer spending on aesthetic and regenerative therapies support steady uptake there. Mature markets in the U.S. and Canada drive demand for both cosmetic and medical applications of injectables. Europe and emerging regions such as Latin America, Middle East & Africa also show growing interest. Europe records steady growth due to increasing acceptance of non-surgical treatments and rising skin-health awareness. Emerging regions adopt injectables gradually, aided by rising disposable incomes, expanding cosmetic clinics, and broader access to global products.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- PharmaResearch
- Bioplus Co., Ltd.
- AMEELA
- Mastelli
- Fox Pharma
- BR Pharm
- DermaFocus
- LG Chem
- Pluryal
- Promoitalia Laboratories
Competitive Analysis:
The polynucleotides injectable industry remains moderately concentrated, with several specialized firms competing globally. Leading firms such as AMEELA, Croma Pharma, Bioplus, and Fox Pharma hold notable shares by offering validated, high-purity DNA-polymer formulations. These companies invest in R&D, clinical trials, and regulatory compliance to differentiate their products on safety and efficacy. They compete on formulation purity, delivery mechanism design, and geographic reach. New entrants face barriers including stringent regulatory approvals and high cost of validation. Some smaller players attempt to differentiate via niche applications like veterinary regenerative care or orthopedic soft-tissue repair. The competitive landscape thus balances between established biotech-driven firms and ambitious niche innovators.
Report Coverage:
The research report offers an in-depth analysis based on Application and End Use. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Growing focus on regenerative aesthetic treatments will continue driving adoption across cosmetic and dermatology clinics.
- Rising awareness about skin repair and collagen stimulation will expand usage beyond facial rejuvenation.
- Integration with hybrid therapies such as PRP and HA fillers will strengthen clinical outcomes and patient satisfaction.
- Technological innovations in molecular formulation will enhance product stability, purity, and longevity.
- Expansion of medical aesthetic tourism in Asia-Pacific will attract international demand for premium procedures.
- Broader clinical validation will support new indications in wound care, orthopedics, and post-surgical healing.
- Increased participation of biopharma companies will diversify supply chains and product development pipelines.
- Growing investments in practitioner training and certification will improve procedural quality worldwide.
- Digital education and influencer marketing will accelerate awareness among younger demographics.
- Regulatory alignment and standardization across major markets will ensure faster approvals and sustained global expansion.




