| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Retractable Needle Safety Syringes Market Size 2024 |
USD 6,391.6 million |
| Retractable Needle Safety Syringes Market, CAGR |
4.39% |
| Retractable Needle Safety Syringes Market Size 2032 |
USD 8,992.1 million |
Market Overview:
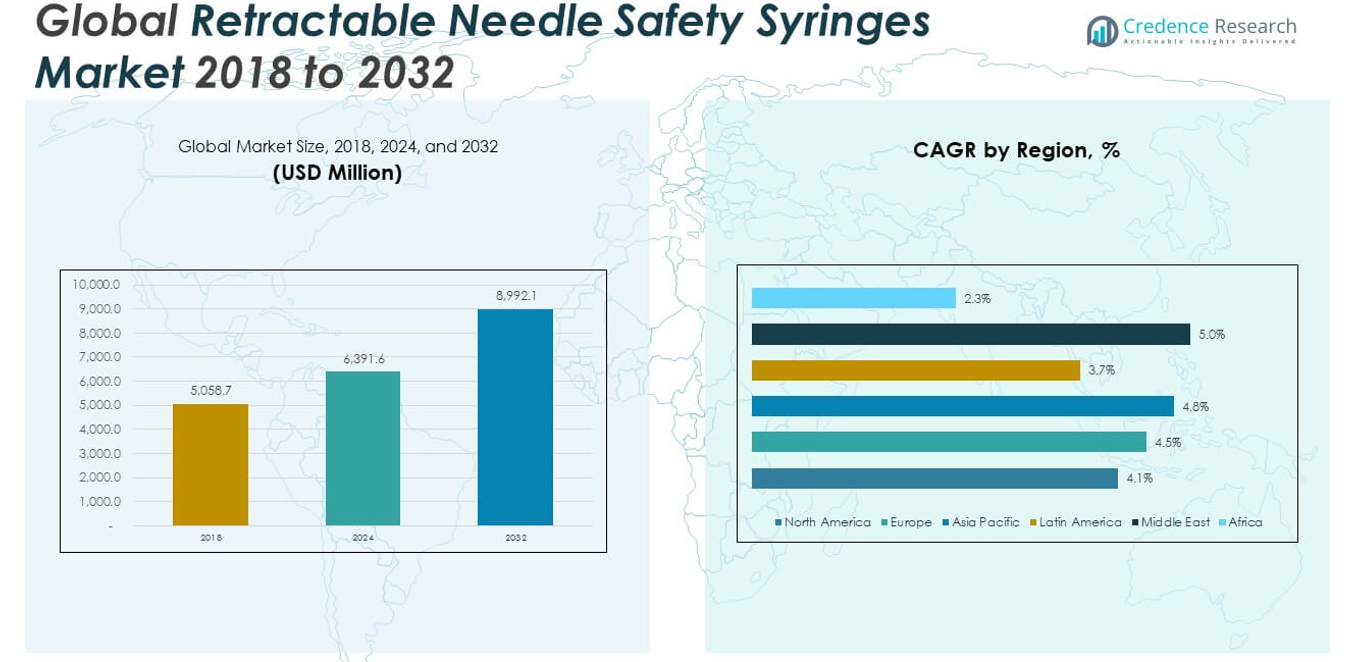
The Global Retractable Needle Safety Syringes Market size was valued at USD 5,058.7 million in 2018 to USD 6,391.6 million in 2024 and is anticipated to reach USD 8,992.1 million by 2032, at a CAGR of 4.39% during the forecast period.
One of the primary drivers of the Global Retractable Needle Safety Syringes Market is the growing incidence of needle-stick injuries (NSIs) and the associated risk of blood-borne infections. Healthcare professionals frequently face exposure to infectious diseases such as hepatitis B, hepatitis C, and HIV due to accidental needle pricks during or after medical procedures. According to the World Health Organization, millions of healthcare workers are exposed to such injuries annually, often due to the reuse of syringes or improper disposal. Retractable needle safety syringes mitigate these risks by automatically withdrawing the needle into the barrel after use, thus eliminating the chances of accidental reuse or contact. Regulatory mandates and occupational safety guidelines from organizations such as OSHA (Occupational Safety and Health Administration) and CDC (Centers for Disease Control and Prevention) have further accelerated adoption. Many national governments have enforced safety syringe use across public healthcare systems, boosting demand in both developed and developing markets.
Regionally, North America leads the Global Retractable Needle Safety Syringes Market, accounting for a significant share due to robust healthcare infrastructure, high procedural volumes, and strict occupational health standards. The United States, in particular, has implemented comprehensive legislation requiring the use of safety-engineered syringes in hospitals and ambulatory care settings, fueling consistent product demand. Europe follows closely, supported by similar regulatory frameworks, increasing healthcare expenditures, and aging populations requiring frequent medical interventions. Countries such as Germany, France, and the U.K. continue to promote the use of advanced injection safety devices through centralized procurement and awareness initiatives. The Asia-Pacific region is emerging as the fastest-growing market, driven by large-scale immunization campaigns, rapid healthcare infrastructure development, and increasing investments in infection control. Nations like China, India, and Indonesia are adopting retractable syringes to strengthen patient and worker safety in densely populated healthcare environments. Government-backed initiatives to eliminate syringe reuse and curb hospital-acquired infections also support growth. Latin America and the Middle East & Africa, though growing at a slower pace, are expected to experience rising demand due to international aid programs, healthcare reforms, and broader access to essential medical supplies. Market growth in these regions depends heavily on local regulatory support, public health funding, and supply chain accessibility.
Market Insights:
- The Global Retractable Needle Safety Syringes Market was valued at USD 5,058.7 million in 2018, reached USD 6,391.6 million in 2024, and is expected to grow to USD 8,992.1 million by 2032 at a CAGR of 4.39% during the forecast period.
- Rising incidence of needle-stick injuries (NSIs) and exposure to blood-borne infections like HIV, hepatitis B, and hepatitis C are accelerating demand for retractable needle safety syringes that eliminate post-use contact.
- Regulatory mandates such as the Needlestick Safety and Prevention Act in the U.S. and safety guidelines from OSHA and CDC are driving mandatory adoption across public and private healthcare sectors.
- Ongoing global vaccination programs for diseases like polio, COVID-19, influenza, and hepatitis are increasing demand for high-volume, single-use retractable syringes to ensure safety in mass immunization campaigns.
- Growing focus on infection control and hospital quality standards is leading healthcare providers to adopt retractable syringes that prevent reuse and improper disposal, aligning with international safety protocols.
- High production costs due to advanced materials and mechanisms are limiting affordability and adoption in low-income countries, where budget constraints impact procurement decisions despite recognized safety benefits.
- North America holds the largest market share due to robust healthcare infrastructure and strict safety laws, Europe follows with centralized procurement efforts, and Asia-Pacific is the fastest-growing region due to healthcare expansion and government-backed safety initiatives in countries like China, India, and Indonesia.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Incidence of Needle-Stick Injuries Among Healthcare Workers Increases Safety Syringe Adoption:
Needle-stick injuries (NSIs) pose a serious occupational hazard for healthcare professionals worldwide. These incidents expose workers to blood-borne pathogens such as HIV, hepatitis B, and hepatitis C. The Global Retractable Needle Safety Syringes Market is gaining momentum due to the ability of these syringes to prevent accidental needle contact post-injection. It supports clinical safety by eliminating manual needle handling and reducing reuse risks. Healthcare facilities are prioritizing safety-engineered devices to comply with workplace safety mandates and minimize liability. This trend reinforces the shift toward retractable syringe systems in hospitals, clinics, and ambulatory care settings.
- For instance, after the introduction of the BD Eclipse™ safety needle, hospitals reported a 64% reduction in needlestick injuries (NSIs), with some studies documenting up to a 100% reduction in NSIs from hypodermic injections. The BD Eclipse™ features a safety shield that allows for one-handed activation, covering the needle immediately after use and confirming activation with an audible click, supporting safer clinical practice and reducing accidental exposure to blood-borne pathogens.
Government Regulations and Institutional Safety Policies Drive Mandatory Usage:
Regulatory bodies have mandated the use of safety syringes to protect healthcare personnel and patients from avoidable injuries and infections. In countries like the United States, legislation such as the Needlestick Safety and Prevention Act requires the implementation of safety-engineered sharps. The Global Retractable Needle Safety Syringes Market benefits from these regulatory frameworks, which accelerate product adoption across public and private healthcare sectors. It aligns with institutional policies designed to improve compliance with occupational safety standards. Procurement authorities and health ministries are including retractable safety syringes in national medical supply lists. This policy-driven momentum enhances long-term demand stability across regions.
- For instance, following the enforcement of the Needlestick Safety and Prevention Act in the United States, healthcare institutions widely adopted BD SafetyGlide™ and BD Eclipse™ safety needles.
Expansion of Global Vaccination Programs Increases Demand for Safe Injection Devices:
Mass immunization efforts have grown worldwide, fueled by disease eradication initiatives and pandemic response strategies. Programs targeting polio, influenza, COVID-19, and hepatitis create sustained demand for high-volume, safe, single-use syringes. The Global Retractable Needle Safety Syringes Market responds to this need by offering devices that reduce transmission risks during mass vaccination drives. It ensures injection safety while supporting large-scale public health logistics. Global health organizations and donor agencies are promoting retractable syringe use to improve immunization safety in low-resource settings. Their adoption is critical for reaching health goals without compromising staff protection.
Increased Focus on Infection Control and Healthcare Quality Boosts Adoption:
Healthcare systems are investing more in infection control technologies to reduce hospital-acquired infections and improve patient safety metrics. Syringes that retract the needle after administration prevent reuse and improper disposal, supporting infection prevention protocols. The Global Retractable Needle Safety Syringes Market aligns with these healthcare quality objectives, offering solutions that integrate safety and compliance. It allows providers to demonstrate adherence to international health standards and improve care outcomes. Infection control committees and quality assurance teams are key influencers in purchasing decisions. Their growing role supports institutional shifts toward standardized use of safety syringes.
Market Trends:
Growing Preference for Passive Retraction Mechanisms Enhances Usability and Compliance:
The market is seeing a shift from manually activated to passive retraction mechanisms that engage automatically after drug administration. These designs eliminate the need for user intervention, improving both ease of use and safety compliance. Healthcare workers prefer passive systems because they reduce human error and standardize post-injection needle containment. The Global Retractable Needle Safety Syringes Market is incorporating these features into new product lines to address growing demands from hospitals and clinics. It enables a streamlined workflow and improves protection without increasing training burden. Passive systems are gaining traction in high-volume care environments where speed and safety must coexist.
- For instance, VanishPoint® syringes by Retractable Technologies, Inc. feature an automated retraction mechanism that virtually eliminates exposure to contaminated needles. The needle is automatically retracted into the barrel of the syringe when the plunger is fully depressed, requiring no additional steps and enabling single-handed activation, which significantly reduces the risk of needlestick injuries for healthcare worker.
Adoption of Sustainable and Eco-Friendly Syringe Materials Gains Momentum:
Environmental considerations are influencing product development in medical devices, including disposable syringes. Manufacturers are moving toward recyclable plastics and biodegradable components to reduce medical waste and meet sustainability goals. The Global Retractable Needle Safety Syringes Market is beginning to adopt these materials to align with healthcare system efforts to reduce their carbon footprint. It also responds to procurement policies that increasingly prioritize environmentally responsible products. Governments and NGOs are advocating for sustainable procurement practices in public health supply chains. This trend encourages innovation in both material science and product lifecycle management.
- For instance, Terumo’s SurGuard®3 Safety Needle has been verified for biocompatibility and safety by the FDA and is manufactured using blood-contacting materials that meet international biological evaluation standards.
Increased Integration with Pre-Filled Syringe Systems Streamlines Drug Delivery:
Pharmaceutical companies are collaborating with syringe manufacturers to develop pre-filled, retractable safety syringes that combine convenience with security. These integrated systems reduce preparation time, minimize contamination risk, and simplify medication administration. The Global Retractable Needle Safety Syringes Market is expanding into this segment to meet the growing demand for ready-to-use injection formats in vaccines, biologics, and chronic therapies. It supports pharmaceutical supply chain efficiency and enhances patient safety in outpatient and self-administration settings. Demand is rising across both clinical and homecare channels, driving broader market reach.
Rising Use of Automation and Robotics in Syringe Manufacturing Improves Scale and Quality:
To meet increasing global demand and maintain high production standards, manufacturers are investing in automation and robotics across syringe production lines. Advanced assembly, sterilization, and quality control systems allow for greater throughput and consistency. The Global Retractable Needle Safety Syringes Market benefits from these investments, which reduce costs and minimize product variability. It also enables rapid scaling during public health emergencies and large procurement cycles. Automated systems improve manufacturing agility and support continuous innovation in device design and function. This trend positions producers to meet both regulatory expectations and commercial growth targets.
Market Challenges Analysis:
High Production Costs and Pricing Constraints Limit Widespread Adoption in Low-Resource Settings:
The advanced design and engineering behind retractable needle safety syringes often lead to higher production costs compared to conventional syringes. Materials, spring mechanisms, and quality assurance processes raise the price point, making these products less accessible in low-income regions. Public health systems and budget-restricted hospitals may hesitate to adopt these devices without sufficient reimbursement or external funding. The Global Retractable Needle Safety Syringes Market must navigate the tension between innovation and affordability to achieve broader penetration. It faces the challenge of balancing safety performance with cost-effectiveness in procurement-driven environments. Price sensitivity continues to affect purchasing decisions, especially in large-scale immunization campaigns.
Lack of Standardization and Training Gaps Undermine Effective Implementation:
Variation in safety syringe designs and activation mechanisms can create confusion among healthcare professionals, leading to inconsistent usage. Without adequate training, users may fail to properly activate retraction features, reducing the intended safety benefits. The Global Retractable Needle Safety Syringes Market faces a critical need to support education and standardization across healthcare systems. It requires alignment with institutional protocols and integration into clinical workflows to ensure effective use. In regions with limited healthcare infrastructure, the absence of user training programs further weakens adoption. Addressing these issues is vital to realizing the full preventive potential of safety-engineered syringes.
Market Opportunities:
Expansion of National Immunization Programs and Donor-Funded Health Initiatives Offers Growth Potential:
Governments and global health organizations continue to scale up immunization efforts, creating strong demand for safe, single-use injection devices. Programs supported by WHO, GAVI, and UNICEF are prioritizing retractable syringes to prevent reuse and improve injection safety. The Global Retractable Needle Safety Syringes Market can leverage this shift by supplying high-volume, cost-effective models tailored for mass administration. It stands to benefit from long-term procurement contracts and global distribution partnerships. Targeted outreach in lower-income regions can increase product visibility and build trust among health ministries and NGOs. This opportunity supports both public health outcomes and commercial expansion.
Adoption in Homecare and Self-Administration Settings Expands End-User Base:
The growing trend of chronic disease management outside hospital environments is driving demand for user-friendly and safe injection devices. Patients managing diabetes, arthritis, or hormone therapies at home require syringes that minimize risk and simplify handling. The Global Retractable Needle Safety Syringes Market can address this need with intuitive, low-force activation systems and ergonomic designs. It enables pharmaceutical companies to bundle these devices with injectable therapies for enhanced convenience. As homecare adoption increases, manufacturers can develop differentiated offerings to meet the specific needs of non-clinical users. This shift opens a promising segment beyond institutional care.
Market Segmentation Analysis:
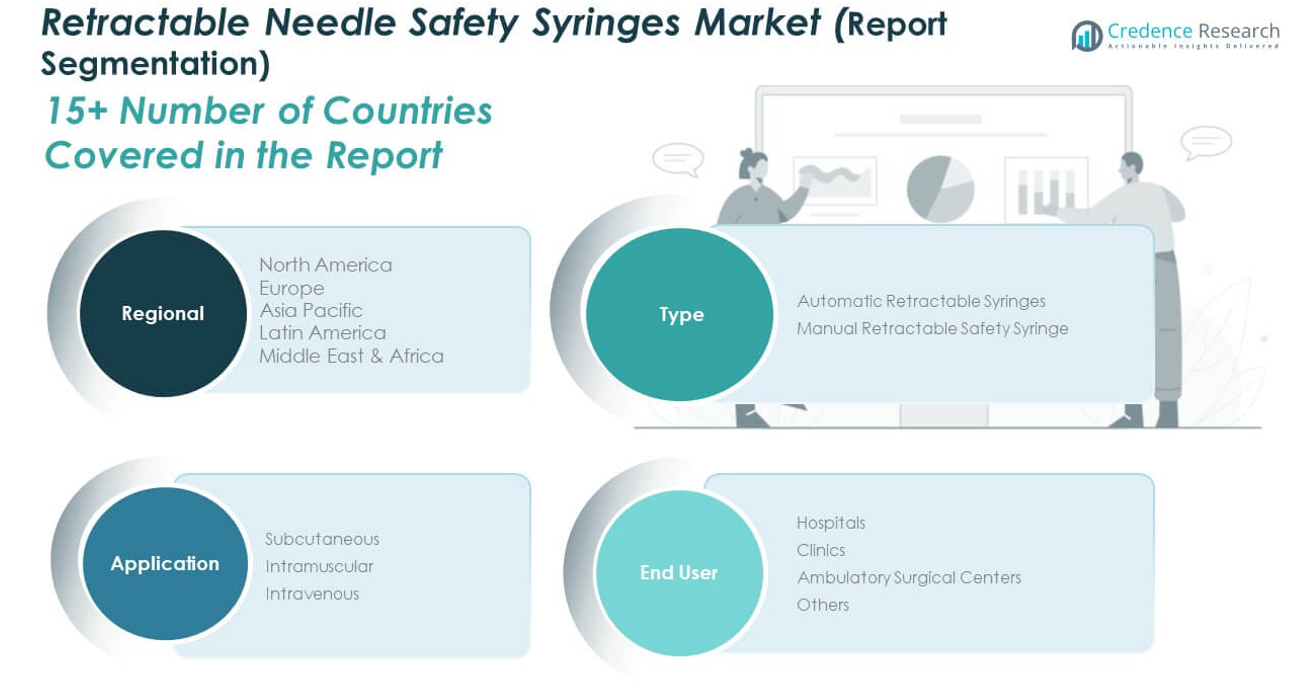
By Type
Automatic retractable syringes hold the dominant market share due to their user-friendly mechanism, reduced risk of needle-stick injuries, and high safety compliance. These syringes are preferred in high-volume healthcare settings where efficiency and patient safety are critical. Manual retractable safety syringes also play a vital role, particularly in resource-limited environments, where they offer a cost-effective yet reliable solution for safe injection practices.
- For instance, Automatic retractable syringes, such as the VanishPoint® series, are recognized for their user-friendly design and ability to virtually eliminate needlestick injuries through automated, passive retraction technology, making them the preferred choice in high-volume healthcare environments.
By Application
Subcutaneous injections account for a substantial share of the market, driven by their widespread use in diabetes care, hormone therapy, and routine immunizations. Intramuscular applications follow, supported by their relevance in emergency care and vaccination campaigns. Intravenous usage, although comparatively lower, remains essential for drug delivery in clinical and acute care settings, where infection prevention is paramount.
- For instance, BD Eclipse™ and BD SafetyGlide™ safety needles are widely used for subcutaneous and intramuscular injections, with studies showing a significant reduction in NSIs across multiple clinical settings after their adoption, supporting safer administration of routine immunizations, diabetes care, and emergency interventions.
By End User
Hospitals lead the end-user segment, backed by regulatory enforcement, high patient throughput, and institutional focus on safety devices. Clinics are experiencing increased adoption of retractable syringes, especially in outpatient care and diagnostic procedures. Ambulatory surgical centers represent a fast-growing segment due to the shift toward same-day procedures and heightened infection control measures. The others segment, including home healthcare and diagnostic labs, adds to the market’s scope by catering to personalized and decentralized care.
Segmentation:
By Type
- Automatic Retractable Syringes
- Manual Retractable Safety Syringe
By Application
- Subcutaneous
- Intramuscular
- Intravenous
By End User
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America
The North America Retractable Needle Safety Syringes Market size was valued at USD 837.72 million in 2018 to USD 1,043.11 million in 2024 and is anticipated to reach USD 1,438.73 million by 2032, at a CAGR of 4.1% during the forecast period. North America holds approximately 16.3% of the global market share. The Global Retractable Needle Safety Syringes Market sees strong growth in this region due to stringent occupational safety regulations and the widespread implementation of safety-engineered devices across healthcare systems. The U.S. drives most of the regional demand, supported by federal mandates like the Needlestick Safety and Prevention Act and extensive use in hospital and outpatient care. High awareness of blood-borne infection risks and investments in healthcare infrastructure further support market penetration. Canada and Mexico contribute through public health campaigns and improved healthcare accessibility. The presence of major manufacturers and favorable reimbursement structures ensures continued product adoption.
Europe
The Europe Retractable Needle Safety Syringes Market size was valued at USD 1,101.78 million in 2018 to USD 1,398.12 million in 2024 and is anticipated to reach USD 1,978.26 million by 2032, at a CAGR of 4.5% during the forecast period. Europe accounts for nearly 22% of the global market share. The market benefits from harmonized regulatory frameworks and centralized procurement programs across member countries. The Global Retractable Needle Safety Syringes Market in Europe is supported by aging populations, higher chronic disease prevalence, and institutional focus on infection prevention. Countries like Germany, France, and the UK lead adoption through public healthcare investments and consistent demand from hospitals and clinics. Regional governments actively promote safe injection practices through policies and funding. Cross-border collaboration for healthcare safety further improves uptake and compliance.
Asia Pacific
The Asia Pacific Retractable Needle Safety Syringes Market size was valued at USD 1,776.11 million in 2018 to USD 2,295.87 million in 2024 and is anticipated to reach USD 3,327.07 million by 2032, at a CAGR of 4.8% during the forecast period. Asia Pacific holds the largest share of the Global Retractable Needle Safety Syringes Market, contributing over 37%. The region is experiencing rapid healthcare infrastructure development and expanding immunization programs across densely populated countries. China and India lead regional demand due to government-backed infection control initiatives and increased healthcare spending. Japan, South Korea, and Southeast Asia follow with rising procedural volumes and technological adoption in hospitals. Donor-supported vaccination campaigns further drive consumption of safety syringes. Local production capacity is growing, improving supply chain efficiency.
Latin America
The Latin America Retractable Needle Safety Syringes Market size was valued at USD 442.64 million in 2018 to USD 538.72 million in 2024 and is anticipated to reach USD 719.37 million by 2032, at a CAGR of 3.7% during the forecast period. Latin America represents around 8% of the global market share. The Global Retractable Needle Safety Syringes Market in this region is shaped by expanding public healthcare access and gradual regulatory alignment with international safety standards. Brazil and Argentina are key contributors, benefiting from national immunization programs and rising awareness among healthcare professionals. Market adoption is slower in smaller economies due to cost constraints and uneven distribution systems. International aid programs and NGO support play a role in market growth. Long-term expansion depends on government funding and local manufacturing capabilities.
Middle East
The Middle East Retractable Needle Safety Syringes Market size was valued at USD 539.76 million in 2018 to USD 705.54 million in 2024 and is anticipated to reach USD 1,036.79 million by 2032, at a CAGR of 5.0% during the forecast period. The region accounts for nearly 12% of the Global Retractable Needle Safety Syringes Market. It benefits from increasing healthcare investments and strong emphasis on patient and occupational safety in GCC countries. Saudi Arabia and the UAE lead demand, supported by hospital upgrades and adherence to international quality protocols. Turkey and Israel also contribute through advanced healthcare systems and public-private partnerships. The market sees growing involvement from global suppliers and regional distributors. Regulatory reforms and population health programs support further penetration.
Africa
The Africa Retractable Needle Safety Syringes Market size was valued at USD 360.69 million in 2018 to USD 410.25 million in 2024 and is anticipated to reach USD 491.87 million by 2032, at a CAGR of 2.3% during the forecast period. Africa holds about 5% of the global market share. The Global Retractable Needle Safety Syringes Market in this region is driven by donor-funded health initiatives and international partnerships aimed at reducing blood-borne infections. Countries like South Africa and Egypt are making gradual progress through policy support and improved healthcare delivery. Adoption is limited in rural areas due to infrastructure and budgetary challenges. NGOs and global health agencies remain essential in improving access to safety syringes. Long-term market growth depends on healthcare reforms and increased domestic investment.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Axel Bio Corporation, Inc
- BD
- Numedico
- Lifelong Group
- DMC Medical Ltd.
- Retractable Technologies, Inc.
- Sol-Millennium, Inc.
- Medigard Limited
- UltiMed, Inc.
- Haiou Medical
- Other Key Players
Competitive Analysis:
The Global Retractable Needle Safety Syringes Market features a competitive landscape shaped by innovation, regulatory compliance, and pricing strategies. Key players include BD (Becton, Dickinson and Company), Retractable Technologies Inc., Terumo Corporation, Smiths Medical, and Medline Industries. These companies compete through product differentiation, patent portfolios, and supply agreements with government and international health organizations. The market includes both global leaders and emerging regional firms offering cost-effective alternatives. It favors manufacturers that balance advanced safety features with scalability and cost-efficiency. The Global Retractable Needle Safety Syringes Market rewards companies that invest in R&D, automation, and user-centric designs. Competitive advantage often depends on securing high-volume contracts, ensuring consistent regulatory approval, and meeting diverse end-user needs across hospitals, clinics, and homecare. Partnerships with pharmaceutical firms and integration with pre-filled drugs represent another key strategic focus for long-term positioning.
Recent Developments:
- In May 2025, Retractable Technologies, Inc. reported continued market presence with its VanishPoint® and Patient Safe® safety medical products, including syringes, blood collection, and IV catheter devices. The VanishPoint® syringe is specifically engineered to prevent needlestick injuries and product reuse by retracting the needle directly from the patient into the barrel, effectively reducing exposure to contaminated needles. The company’s products are distributed through multiple specialty and general line distributors.
- In 2025, Axel Bio Corporation, Inc. reaffirmed its focus on supplying FDA 510(k) and CE-marked retractable safety syringes to healthcare providers. The company emphasizes compliance with federal safety guidelines and delivers high-quality, cost-effective medical devices designed to reduce needle-stick injuries among healthcare professionals. Axel Bio’s retractable safety syringes are part of a broader portfolio that includes PPE and sterilization products for infection control.
Market Concentration & Characteristics:
The Global Retractable Needle Safety Syringes Market shows moderate concentration, with a few dominant players controlling significant share through established brands, regulatory expertise, and global distribution networks. It is characterized by high compliance requirements, technological specialization, and demand for scalable manufacturing. The market balances innovation with affordability, appealing to both high-income and resource-limited settings. It supports a mix of manual and automatic retraction mechanisms tailored to diverse clinical and homecare needs. Product differentiation relies on usability, safety design, and compatibility with drug delivery formats. The Global Retractable Needle Safety Syringes Market continues to evolve through material advancements, sustainability initiatives, and partnerships with public health agencies.
Report Coverage:
The research report offers an in-depth analysis based on by type, application, and end user. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Rising global focus on healthcare worker safety will increase demand for retractable syringes.
- Expansion of vaccination programs will create consistent high-volume procurement opportunities.
- Adoption of passive retraction mechanisms will drive design innovation and ease of use.
- Growth in self-administration and homecare will expand the non-clinical end-user base.
- Regulatory support and mandates will continue to accelerate institutional adoption worldwide.
- Integration with pre-filled injectable therapies will offer new revenue streams for manufacturers.
- Investments in automated manufacturing will improve production scalability and reduce costs.
- Sustainability efforts will push development of recyclable and eco-friendly syringe materials.
- Emerging markets will contribute significantly through healthcare infrastructure upgrades.
- Strategic collaborations with global health organizations will strengthen market penetration and distribution.





