Market Overview
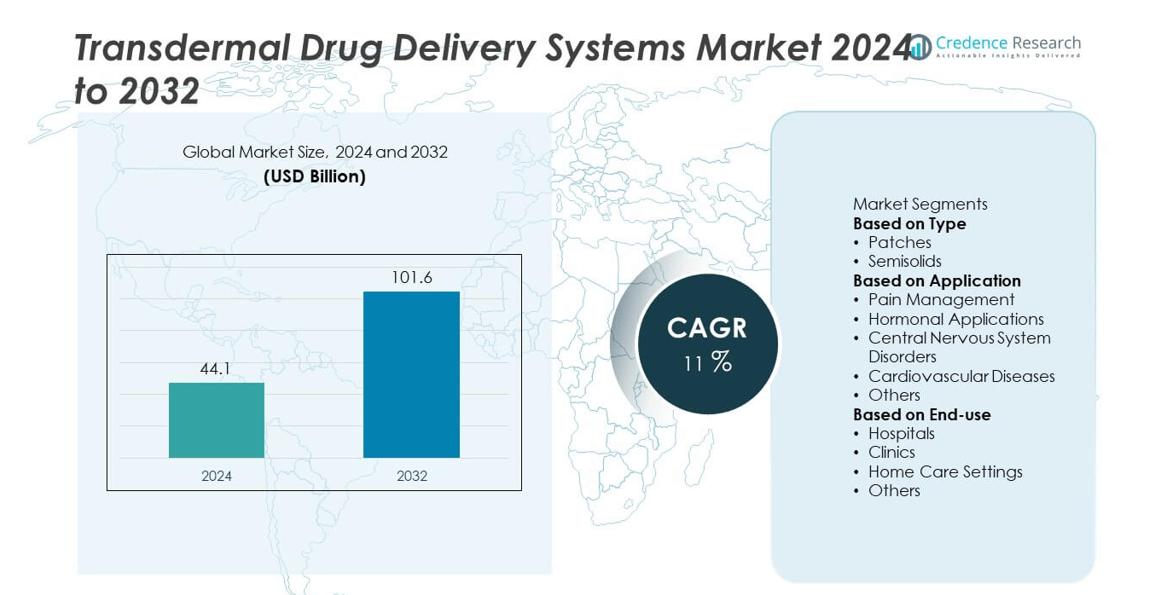
The Transdermal Drug Delivery Systems Market was valued at USD 44.1 billion in 2024 and is projected to reach USD 101.6 billion by 2032, expanding at a CAGR of 11% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Transdermal Drug Delivery Systems Market Size 2024 |
USD 44.1 billion |
| Transdermal Drug Delivery Systems Market, CAGR |
11% |
| Transdermal Drug Delivery Systems Market Size 2032 |
USD 101.6 billion |
The Transdermal Drug Delivery Systems Market grows with rising demand for non-invasive, patient-friendly therapies that improve compliance in chronic disease management. Increasing prevalence of conditions such as diabetes, cardiovascular disorders, and neurological diseases strengthens reliance on patches and gels for sustained drug release.
The Transdermal Drug Delivery Systems Market demonstrates strong regional diversity, with North America leading adoption due to advanced healthcare infrastructure and strong acceptance of innovative therapies. Europe follows with significant demand supported by an aging population and patient-friendly treatment policies, while Asia-Pacific emerges as the fastest-growing region driven by healthcare investments in China, Japan, and India. Latin America and the Middle East & Africa gradually expand adoption as access to modern therapies improves in urban centers. Key players shaping this market include Novartis, Hisamitsu Pharmaceutical Co Inc, Corium Inc, and Luye Pharma. These companies focus on expanding transdermal product portfolios, improving adhesive technologies, and introducing smart wearable patches for chronic conditions. It creates a competitive landscape defined by continuous research, strong regulatory engagement, and increasing partnerships to address unmet medical needs. Regional opportunities further strengthen prospects for both established players and emerging firms.
Market Insights
- The Transdermal Drug Delivery Systems Market was valued at USD 44.1 billion in 2024 and is projected to reach USD 101.6 billion by 2032, expanding at a CAGR of 11% during the forecast period.
- Growing prevalence of chronic conditions such as diabetes, cardiovascular disorders, and neurological diseases drives demand for non-invasive, patient-friendly therapies that ensure higher compliance and improved treatment outcomes.
- Advancements in microneedle patches, sensor-enabled wearables, and permeation enhancers create strong innovation momentum, expanding applications to biologics, vaccines, and complex drug molecules.
- Competitive activity remains high, with key players such as Novartis, Hisamitsu Pharmaceutical Co Inc, Corium Inc, and Luye Pharma focusing on expanding patch portfolios, securing regulatory approvals, and investing in digital health integration.
- High manufacturing costs, limited suitability for large-molecule drugs, and stringent regulatory requirements restrain rapid product expansion, especially for smaller and mid-sized firms with fewer resources.
- North America leads adoption due to robust infrastructure, favorable reimbursement policies, and advanced R&D capabilities, while Europe follows with strong demand in chronic and neurological care.
- Asia-Pacific emerges as the fastest-growing region, supported by rising healthcare investments in China, India, and Japan, while Latin America and the Middle East & Africa gradually expand adoption through urban healthcare networks and increased private sector participation.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Rising Prevalence of Chronic Diseases and Need for Non-Invasive Therapies
The Transdermal Drug Delivery Systems Market benefits from the global rise in chronic illnesses such as diabetes, cardiovascular disorders, and neurological conditions. Patients seek alternatives to frequent injections and oral medications that often create compliance issues. Transdermal systems provide sustained drug release and reduce gastrointestinal side effects. Physicians increasingly recommend these therapies for long-term treatment management. It supports improved patient adherence and overall treatment efficiency. Growing use across diverse therapeutic areas positions the market for consistent demand.
- For instance, Corium Inc. launched its Adlarity® donepezil patch, which provides continuous drug release over 7 days for patients with Alzheimer’s disease. This once-weekly application reduces the pill burden and offers a favorable gastrointestinal side effect profile
Advancements in Drug Formulation and Delivery Technologies
Continuous progress in drug formulation methods drives the growth of the Transdermal Drug Delivery Systems Market. Enhanced adhesive properties ensure patches remain effective for extended durations without skin irritation. Microneedle-based patches improve the bioavailability of complex drugs and vaccines. Wearable systems equipped with sensors enable controlled and personalized dosing. It expands opportunities for biologics and large-molecule drugs once restricted to injections. Pharmaceutical companies invest heavily in R&D to introduce next-generation transdermal solutions.
- For instance, Hisamitsu’s Salonpas patch was named the world’s No. 1 OTC topical analgesic patch brand for the ninth consecutive year as of May 16, 2025, based on research by Euromonitor International. This recognition is a result of retail sales data comparisons across thirteen countries and regions representing a significant portion of the global topical patch market.
Increasing Focus on Patient Convenience and Compliance
The Transdermal Drug Delivery Systems Market gains traction through its ability to simplify treatment schedules. Patches and advanced devices reduce dosing frequency, which helps patients avoid missed medication cycles. Convenience drives adoption among elderly populations and those with complex treatment regimens. Healthcare providers emphasize solutions that enhance compliance rates and improve clinical outcomes. It creates strong acceptance among patients preferring discreet and non-invasive therapies. Rising awareness about lifestyle-friendly treatment options strengthens demand across multiple regions.
Supportive Regulatory Approvals and Expanding Pharmaceutical Pipeline
The Transdermal Drug Delivery Systems Market benefits from favorable regulatory frameworks supporting innovative therapies. Agencies approve an increasing number of transdermal patches for varied indications. Expanding pharmaceutical pipelines now include drugs specifically designed for transdermal delivery. Regulatory bodies encourage advancements that improve patient safety and therapeutic efficiency. It promotes faster commercialization of new products across key markets. Strong alignment between regulators and industry stakeholders accelerates innovation and drives sustained growth potential.
Market Trends
Growing Adoption of Microneedle Patches and Smart Wearable Devices
The Transdermal Drug Delivery Systems Market experiences growth through the introduction of microneedle patches and sensor-based devices. These innovations enable painless and precise drug administration while improving user comfort. Smart wearables track adherence and deliver controlled doses, creating stronger value in chronic disease care. Companies expand offerings for insulin, vaccines, and hormonal therapies using such platforms. It drives interest among healthcare providers seeking advanced patient-friendly solutions. Rising investment in next-generation wearables positions this trend as a core growth factor.
- For instance, Micropoint Technologies has developed dissolvable microneedle patches, such as the MPatch™ Mini, which use over one hundred biodegradable needles per patch. The company has conducted clinical evaluations showing safety and effectiveness for scar treatment, and is actively seeking partners for future applications, including vaccine administration.
Expansion of Biologics and Vaccines Through Transdermal Delivery Platforms
Biologics and vaccines increasingly utilize transdermal platforms to enhance stability and simplify delivery. The Transdermal Drug Delivery Systems Market benefits from formulations that bypass gastrointestinal degradation. Microporous patches and bioengineered carriers make delivery of large-molecule drugs feasible. Expanding use in influenza, COVID-19, and other vaccine applications reflects this trend. It supports mass immunization programs with reduced reliance on needles. Growing pharmaceutical partnerships advance the pipeline of biologics suitable for transdermal systems.
- For instance, Zydus Pharmaceuticals introduced its rivastigmine transdermal system in 4.6 mg and 9.5 mg daily doses, while Novartis has conducted clinical studies on microneedle-based influenza patches tested in groups exceeding 100 participants, demonstrating feasibility for vaccine-scale applications.
Rising Demand for Personalized and Controlled Release Solutions
Personalized medicine encourages adoption of systems offering adjustable release rates and patient-specific dosing. The Transdermal Drug Delivery Systems Market aligns with this trend through controlled-release technologies. Wearable systems allow real-time monitoring and adjustments based on patient response. Longer wear times and improved adhesive formulations strengthen treatment consistency. It enables greater precision for conditions requiring stable drug levels over extended periods. Adoption of controlled-release designs expands across therapeutic areas such as pain, neurology, and endocrinology.
Sustainability and Eco-Friendly Patch Development in Manufacturing
Sustainability emerges as a key trend shaping industry direction. The Transdermal Drug Delivery Systems Market witnesses rising focus on biodegradable materials and recyclable patch designs. Manufacturers invest in reducing plastic use and adopting greener production processes. Environment-conscious healthcare systems demand products with lower ecological impact. It reinforces alignment between patient health benefits and environmental goals. Sustainability-driven innovations support brand differentiation and enhance long-term adoption in competitive markets.
Market Challenges Analysis
Complexity of Drug Formulation and Limited Suitability for All Molecules
The Transdermal Drug Delivery Systems Market faces challenges in developing formulations that penetrate the skin effectively. Only certain drugs with specific molecular weights and solubility can be delivered transdermally. Large-molecule biologics and many hydrophilic compounds remain unsuitable for patch-based systems. It limits the potential therapeutic areas despite strong demand for non-invasive methods. Formulation complexity also raises development timelines and costs for pharmaceutical companies. These scientific hurdles restrict the expansion of transdermal technologies across all drug classes.
High Manufacturing Costs and Stringent Regulatory Requirements
Production of advanced transdermal systems involves expensive adhesives, backing layers, and permeation enhancers. The Transdermal Drug Delivery Systems Market struggles with balancing innovation and cost efficiency. Strict regulatory guidelines further increase development expenses through extended testing and compliance measures. It creates barriers for small and mid-sized firms that lack financial resources. Variations in regional approval processes also slow global commercialization. These challenges pressure companies to invest heavily in quality control while ensuring competitive pricing strategies.
Market Opportunities
Expansion into Emerging Therapeutic Areas and Untapped Patient Populations
The Transdermal Drug Delivery Systems Market presents strong opportunities in new therapeutic areas such as oncology, neurology, and hormone replacement. Expanding research supports patch-based solutions for complex conditions that require controlled dosing. Elderly populations and pediatric patients, who often face compliance issues with oral or injectable drugs, represent untapped demand. It creates scope for tailored patch designs that address diverse patient needs. Healthcare systems in emerging economies also expand adoption due to growing awareness of non-invasive options. Targeting these segments allows companies to diversify revenue streams while meeting global treatment demands.
Integration of Digital Health Technologies and Smart Drug Delivery Platforms
Integration of digital health tools opens opportunities for advanced monitoring and personalized therapy. The Transdermal Drug Delivery Systems Market benefits from smart patches that collect real-time patient data and support connected healthcare ecosystems. Remote monitoring solutions enhance adherence and allow physicians to optimize treatment strategies. It increases the value of transdermal systems within modern healthcare frameworks focused on precision medicine. Partnerships between pharmaceutical firms and digital health companies accelerate innovation in this space. Combining drug delivery with data-driven platforms positions transdermal technologies as central to future healthcare models.
Market Segmentation Analysis:
By Type
The Transdermal Drug Delivery Systems Market includes patches and gels as the dominant product categories. Patches hold significant demand due to their convenience, controlled drug release, and extended wear times. Variants such as matrix, reservoir, and microneedle patches expand therapeutic applications beyond pain management into vaccines and chronic disease care. Gels provide flexibility in topical use for dermatology, hormonal therapy, and localized pain relief. It supports adoption among patients preferring non-invasive options that reduce side effects linked to oral medications. Growth in advanced adhesive technologies and permeation enhancers strengthens both patches and gel-based solutions.
- For instance, Luye Pharma markets its once-daily rivastigmine patches that come in dose strengths of 4.6 mg/24 h, 9.5 mg/24 h, and 13.3 mg/24 h, with the highest-dose patch approved by German regulators and available across more than 20 countries.
By Application
Therapeutic applications shape the performance of the Transdermal Drug Delivery Systems Market across several disease areas. Pain management remains the largest application, supported by rising cases of arthritis, back pain, and cancer-related discomfort. Hormone replacement therapy also contributes strong demand due to long-term treatment needs in endocrinology and women’s health. Cardiovascular disorders represent another growth area, with patches offering sustained drug levels to improve compliance. Neurological disorders, including Parkinson’s disease and Alzheimer’s, expand the scope of applications. It creates steady demand across both acute and chronic treatments where consistent dosing is critical.
- For instance, Amneal Pharmaceuticals commercialized clonidine transdermal patches in the U.S. across 0.1 mg, 0.2 mg, and 0.3 mg per day dosing strengths, supporting cardiovascular therapy with continuous seven-day drug release.
By End-use
Hospitals, clinics, and home care settings form the key end-use segments of the Transdermal Drug Delivery Systems Market. Hospitals lead adoption through large patient pools requiring chronic therapy management. Clinics support significant uptake for hormone therapy, dermatology care, and specialized treatments. Home care emerges as a fast-growing segment, with patients favoring self-administration and convenience. It reflects rising demand for products that align with lifestyle needs and reduce the need for frequent hospital visits. Expanding telehealth services and remote monitoring enhance the role of transdermal systems in home-based care. This trend reinforces long-term growth across all end-use categories.
Segments:
Based on Type
Based on Application
- Pain Management
- Hormonal Applications
- Central Nervous System Disorders
- Cardiovascular Diseases
- Others
Based on End-use
- Hospitals
- Clinics
- Home Care Settings
- Others
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America holds the largest share of the Transdermal Drug Delivery Systems Market at 41% in 2024. Strong healthcare infrastructure, high adoption of non-invasive drug delivery, and rising prevalence of chronic conditions support the regional dominance. The United States leads with extensive use of transdermal patches for pain management, hormone replacement, and cardiovascular care. Pharmaceutical firms in the region invest in advanced microneedle patches and sensor-based wearables to expand applications. It benefits from favorable reimbursement frameworks that make therapies accessible to large patient populations. Canada also contributes with increasing acceptance of home-based care and demand for lifestyle-friendly drug delivery systems. Robust regulatory approvals further ensure a steady pipeline of innovative transdermal products.
Europe
Europe accounts for 27% of the Transdermal Drug Delivery Systems Market in 2024. The region demonstrates strong demand across Germany, the United Kingdom, France, and Italy. Healthcare systems promote patient-centered solutions, encouraging adoption of patches for chronic and neurological diseases. It benefits from supportive policies that promote generic transdermal formulations, reducing overall treatment costs. Germany leads with advanced research and manufacturing capabilities, while the United Kingdom emphasizes innovation in biologics delivery. The region’s aging population further drives reliance on therapies designed for compliance and convenience. Expanding clinical trials across EU countries supports growth, particularly in pain and hormone-related treatments.
Asia-Pacific
Asia-Pacific holds 20% of the Transdermal Drug Delivery Systems Market in 2024, emerging as the fastest-growing region. China and Japan lead adoption, supported by strong pharmaceutical manufacturing capacity and rising healthcare investment. India shows rapid uptake, driven by an expanding middle-class population and growing awareness of patient-friendly therapies. It benefits from cost-effective production capabilities, enabling wider regional availability of patches and gels. Japan drives technological leadership, with companies advancing microneedle and biodegradable patch designs. Rising chronic disease prevalence and government focus on affordable healthcare create strong opportunities. The region’s growth trajectory reflects rising demand for innovative yet cost-effective delivery systems.
Latin America
Latin America represents 7% of the Transdermal Drug Delivery Systems Market in 2024. Brazil and Mexico remain the largest contributors, with expanding hospital infrastructure and growing access to chronic disease treatments. Adoption of patches for pain relief and hormone therapy gains traction among urban populations. It faces challenges from uneven healthcare access across rural areas, but increasing private healthcare investments offset these gaps. Brazil strengthens local production and distribution, while Mexico expands imports of advanced systems from North America. Regulatory reforms aim to encourage faster approvals of innovative devices. Rising awareness among patients regarding non-invasive therapies supports steady adoption in the region.
Middle East & Africa
The Middle East & Africa hold 5% of the Transdermal Drug Delivery Systems Market in 2024. Gulf countries, led by Saudi Arabia and the United Arab Emirates, drive adoption with investments in advanced healthcare facilities. South Africa shows gradual uptake of transdermal patches in pain management and infectious disease care. It benefits from initiatives that strengthen access to modern therapies in urban healthcare centers. Limited affordability and restricted healthcare infrastructure in several African nations remain barriers. However, multinational pharmaceutical companies increase their presence to expand accessibility. Regional governments focus on improving patient care options, creating incremental demand for non-invasive drug delivery technologies.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Corium Inc
- Zydus Pharmaceuticals, Inc
- Micropoint Technologies
- Novartis
- Luye Pharma
- Amneal Pharmaceuticals Inc
- Hisamitsu Pharmaceutical Co Inc
- UCB
- Sparsha Pharma International Pvt. Ltd.
- Mylan
Competitive Analysis
Competitive landscape of the Transdermal Drug Delivery Systems Market is defined by strong participation from Novartis, Mylan, Amneal Pharmaceuticals Inc, Zydus Pharmaceuticals Inc, UCB, Hisamitsu Pharmaceutical Co Inc, Corium Inc, Micropoint Technologies, Sparsha Pharma International Pvt. Ltd., and Luye Pharma. These companies focus on expanding product portfolios, securing regulatory approvals, and driving innovation in advanced patch technologies. Competition is shaped by continuous investment in microneedle-based systems, sensor-enabled wearables, and long-duration adhesive solutions designed to improve patient adherence. Global leaders emphasize partnerships and R&D programs that accelerate entry into biologics and vaccine delivery, enhancing growth opportunities across high-demand therapeutic areas. Cost efficiency and scalability remain important strategies, with firms balancing affordability and innovation to capture wider patient bases. Emerging players contribute with niche expertise in eco-friendly designs and region-specific solutions, creating a diverse competitive field. The market demonstrates a mix of established multinationals and regional firms working to address growing demand for non-invasive drug delivery while strengthening global presence through research, licensing, and commercialization activities.
Recent Developments
- In November 2023, AbbVie’s Allergan discontinues Androderm, the exclusive supplier of testosterone transdermal patches, marking the cessation of this product in the market.
- In September 2023, Nutriband declares the receipt of a U.S. patent for its transdermal abuse-deterrent technology, showcasing advancements in preventing misuse through innovative drug delivery methods.
- In September 2023, Satio’s received funding from ARPA-H is a significant milestone in the company’s journey, focusing on the development of SatioRx, a novel transdermal drug delivery device designed for home use and remote control.
Report Coverage
The research report offers an in-depth analysis based on Type, Application, End-use and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Demand for non-invasive therapies will continue to rise across chronic disease management.
- Microneedle patches will gain wider approval and adoption in pain, vaccine, and hormonal therapy.
- Digital health integration will enhance patient monitoring and adherence with smart wearable patches.
- Biologics and large-molecule drugs will expand entry into transdermal delivery platforms.
- Personalized medicine will drive demand for adjustable and controlled-release patch technologies.
- Sustainability initiatives will encourage development of biodegradable and eco-friendly patch materials.
- Regional growth will accelerate in Asia-Pacific supported by healthcare investment and manufacturing capacity.
- Strategic partnerships between pharma companies and tech firms will boost innovation pipelines.
- Regulatory frameworks will increasingly favor rapid approvals of advanced delivery systems.
- Competitive intensity will increase as new entrants focus on affordable and patient-friendly solutions.




