Market Overview:
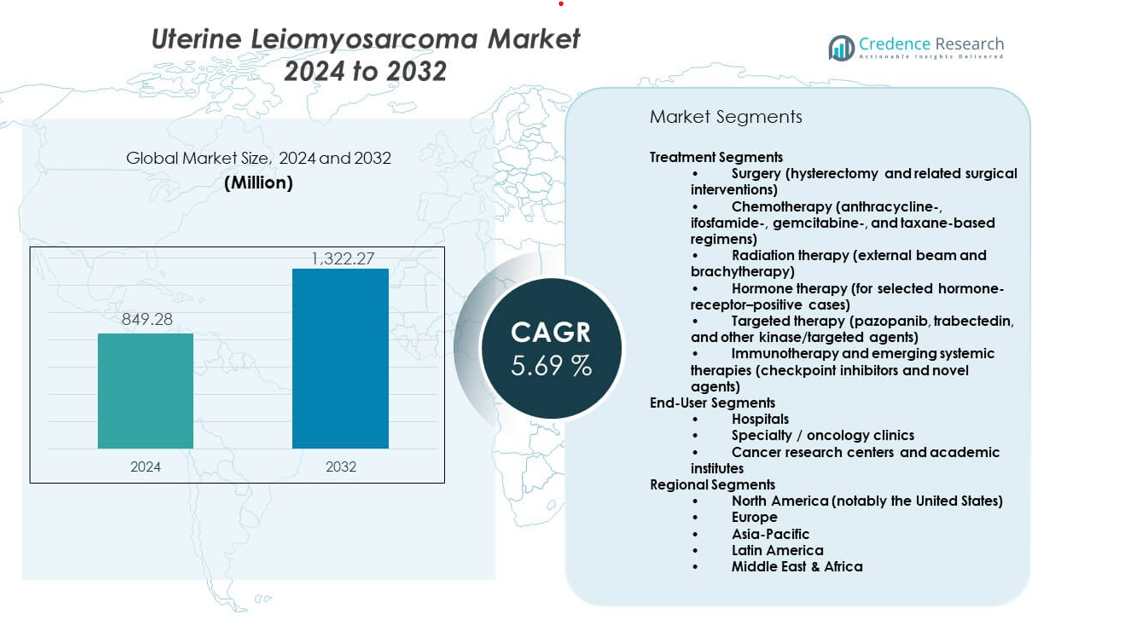
The Uterine Leiomyosarcoma Market is projected to grow from USD 849.28 million in 2024 to an estimated USD 1,322.27 million by 2032, with a compound annual growth rate (CAGR) of 5.69% from 2024 to 2032.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Uterine Leiomyosarcoma Market Size 2024 |
USD 849.28 million |
| Uterine Leiomyosarcoma Market, CAGR |
5.69% |
| Uterine Leiomyosarcoma Market Size 2032 |
USD 1,322.27 million |
Rising treatment demand drives the Uterine Leiomyosarcoma Market due to higher disease awareness and wider use of imaging tools in early detection. Hospitals expand oncology units to handle complex sarcoma cases with stronger precision care. New therapies gain attention due to their role in slowing tumor progression. Research groups focus on improved biomarkers that help doctors refine treatment plans. Drug pipelines grow with novel agents that aim to boost survival rates. Clinical trials attract stronger investment as companies push for better therapeutic options. These factors shape a steady rise in global adoption.
North America leads the Uterine Leiomyosarcoma Market due to strong oncology infrastructure, high diagnostic accuracy, and rapid uptake of advanced therapies. Europe follows with well-structured cancer programs and broad access to specialized hospitals. Asia Pacific emerges fast as healthcare systems expand cancer screening and treatment capacity. China and India upgrade oncology centers due to rising case loads and stronger investment. Latin America and the Middle East show steady progress as countries improve diagnostic pathways and increase access to modern therapeutics. These regions support long-term market expansion.
Market Insights:
- The Uterine Leiomyosarcoma Market is projected to grow from USD 849.28 million in 2024 to USD 1,322.27 million by 2032, registering a CAGR of 5.69%, driven by rising treatment adoption and stronger oncology infrastructure.
- North America (40%), Europe (30%), and Asia-Pacific (20%) hold the top regional shares due to advanced diagnostics, structured cancer programs, and expanding precision-medicine adoption that strengthen overall treatment readiness.
- Asia-Pacific represents the fastest-growing region, supported by its 20% share, rapid oncology-center development, higher screening activity, and increasing access to advanced systemic therapies.
- Surgery accounted for the largest segment share at nearly 35%, driven by its central role in primary tumor management and early-stage intervention across global oncology centers.
- Chemotherapy contributed about 25% share, supported by strong use of anthracycline-, ifosfamide-, gemcitabine-, and taxane-based regimens in standard treatment protocols.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Adoption of Advanced Diagnostic Imaging and Early Detection Tools
Growing use of MRI and CT scans supports early detection in many care systems. Doctors identify tumors at earlier stages due to stronger diagnostic workflow integration. Precision imaging helps oncologists select treatment that aligns with tumor behavior. Hospitals invest in modern scanners to reduce diagnostic gaps across regions. Wider screening programs promote faster referrals to oncology teams for evaluation. Improved accuracy enhances outcomes in the Uterine Leiomyosarcoma Market through timely clinical decisions. It creates stronger confidence among specialists who rely on consistent imaging quality. Rising awareness improves patient engagement during treatment planning.
- For instance, Siemens Healthineers’ MAGNETOM Free.Max offers an 80 cm bore and advanced AI-assisted image reconstruction that improves soft-tissue clarity.
Increasing Focus on Targeted Therapies and Precision Oncology Advancements
Targeted drugs gain interest due to their role in controlling tumor growth with greater accuracy. Researchers refine therapies that interact with molecular pathways linked to disease progression. Pharmaceutical firms invest in platforms that support companion diagnostics development. Oncologists evaluate genetic markers before finalizing drug plans for complex cases. Precision tools improve safety by reducing response variations among patient groups. Clinical teams adopt structured monitoring to track therapy effectiveness in real time. The Uterine Leiomyosarcoma Market benefits from innovation that strengthens patient outcomes. It pushes companies to expand the clinical scope of targeted agents.
- For instance, the PALETTE trial showed that Novartis’ Votrient (pazopanib) achieved a median progression-free survival of 4.6 months compared with 1.6 months with placebo in soft-tissue sarcoma.
Expansion of Oncology Infrastructure and Improved Patient Access to Treatment
Hospitals upgrade oncology units to handle rising patient volume and complex sarcoma care needs. Providers enhance surgical capacity by training teams for high-risk uterine procedures. Cancer centers integrate multidisciplinary boards to guide treatment decisions. Support services expand to help patients manage therapy schedules with fewer delays. Digital tools streamline consultations and improve follow-up evaluation. Treatment facilities invest in specialized staff to reduce survival gaps across regions. The Uterine Leiomyosarcoma Market gains momentum due to infrastructure improvement. It supports broader treatment visibility among underserved populations.
Growing Clinical Research Activity and Strong Pipeline of Investigational Drugs
Clinical trials gain momentum due to high interest in next-generation therapeutics. Investigational agents move into advanced phases with supportive safety data. Research institutions track survival metrics to refine therapy combinations. Sponsors explore drug resistance patterns to strengthen long-term clinical outcomes. Collaboration networks connect hospitals and academic labs for shared trial enrollment. Patient advocacy groups promote awareness that increases participation in key studies. The Uterine Leiomyosarcoma Market benefits from strong scientific focus on novel candidates. It builds a long runway for future therapy launches.
Market Trends:
Shift Toward Immunotherapy Integration in Sarcoma Treatment Pathways
Immunotherapy attracts major interest due to potential in complex uterine sarcomas. Oncologists study immune response behavior to refine dosage strategies. Research teams evaluate checkpoint inhibitors across multiple patient groups. Hospitals explore combination regimens to enhance tumor control durability. Biomarker-based selection supports better identification of responsive cases. Trial networks expand to record real-world outcomes with diverse cohorts. The Uterine Leiomyosarcoma Market gains strength from immunotherapy experimentation. It signals a major shift toward long-term immune modulation strategies.
- For instance, the SARC028 phase II trial reported a 0% objective response rate to pembrolizumab in leiomyosarcoma patients, while a 40% objective response rate was observed in undifferentiated pleomorphic sarcoma patients, highlighting subtype-specific activity.
Rising Use of Real-World Evidence to Guide Clinical Decision-Making
Healthcare systems adopt real-world evidence tools to review therapy performance in daily practice. Data from electronic health records helps recognize unmet needs across populations. Oncologists adjust treatment pathways after observing long-term patient response patterns. Real-world datasets highlight gaps in follow-up care and survival variation. Researchers use analytics platforms to validate outcomes seen in controlled trials. Providers also identify toxicity trends early through population monitoring. The Uterine Leiomyosarcoma Market evolves with stronger data-driven evaluation. It encourages continuous improvement in treatment design.
- For instance, Flatiron Health’s RWE platform aggregates data from more than 3 million U.S. cancer patients, enabling granular evaluation of treatment outcomes.
Growing Role of Genomic Profiling in Treatment Personalization
Genomic testing gains wider use due to decreasing cost and broader availability. Doctors order sequencing tests to identify actionable mutations linked to tumor growth. Molecular labs process samples faster due to automation upgrades. Personalized regimens emerge from detailed genomic interpretation by specialists. Tumor behavior insights help oncologists reduce therapeutic uncertainty. Sequencing adoption expands across hospitals with modern diagnostic setups. The Uterine Leiomyosarcoma Market benefits from strong alignment with precision medicine. It supports long-term improvements in individualized therapy planning.
Expansion of Digital Health Tools to Support Oncology Workflow Efficiency
Digital platforms help hospitals streamline patient scheduling across complex oncology pathways. Tele-oncology supports remote consultations for follow-up care and symptom management. Electronic prescribing reduces documentation errors in multi-drug regimens. Automated alerts guide clinicians during therapy adjustments and toxicity monitoring. Care teams rely on digital dashboards to track patient outcomes over time. Treatment mapping tools improve coordination between surgeons, oncologists, and support staff. The Uterine Leiomyosarcoma Market benefits from these digital upgrades that enhance workflow. It leads to better patient experience and faster decision cycles.
Market Challenges Analysis:
Limited Awareness, Late Diagnosis Patterns, and Complex Disease Behavior
Low awareness levels cause delayed diagnosis in many regions with limited oncology access. Tumors often progress before patients seek specialized care from trained teams. Treatment complexity increases due to aggressive behavior linked to disease biology. Specialists struggle with limited biomarkers that guide accurate personalization. Hospitals face difficulty managing cases that require high-risk surgical procedures. Many regions lack multidisciplinary resources that support advanced oncology planning. The Uterine Leiomyosarcoma Market feels the strain from uneven clinical readiness. It reflects high variability in global care quality.
Constrained Therapy Availability, High Treatment Cost, and Limited Outcome Predictability
Access to advanced therapeutics remains uneven across many healthcare environments. Patients struggle with financial burden tied to high-cost drug regimens. Drug availability issues restrict treatment continuity in lower-resource regions. Oncologists face uncertainty regarding long-term outcome predictability in rare tumor types. Researchers also face enrollment hurdles that slow clinical testing progress. Regulatory review cycles extend approval timelines for new therapies. The Uterine Leiomyosarcoma Market navigates these barriers in growth and innovation. It highlights the need for stronger support frameworks.
Market Opportunities:
Rising Expansion of Precision Medicine Platforms and Development of Novel Therapies
Precision medicine improves treatment clarity through biomarker-guided therapy selection. Genomic profiling identifies new targets for next-generation drug pipelines. Biotech firms invest in therapies that address immune pathways linked to tumor behavior. Hospitals adopt sequencing tools that support better evaluation of rare cancers. International collaborations expand access to clinical trials for underserved populations. The Uterine Leiomyosarcoma Market gains opportunities from scientific innovation. It drives long-term potential for transformative therapeutic choices. Stakeholders accelerate investments in advanced oncology tools.
Growing Scope for Global Screening Programs and Strengthening of Oncology Infrastructure
Governments expand cancer screening initiatives to encourage earlier detection among women. Healthcare systems invest in comprehensive oncology hubs with specialized surgical teams. Training programs strengthen clinical readiness for rare sarcoma treatment. Screening frameworks improve referral speed across primary and tertiary care networks. Providers adopt rapid diagnostic tools that enhance accuracy in tumor assessment. The Uterine Leiomyosarcoma Market benefits from global infrastructure improvement. It builds strong momentum for improved patient outcomes. Growth potential rises with wider access to structured care pathways.
Market Segmentation Analysis:
Treatment Segments
The Uterine Leiomyosarcoma Market expands through a diverse set of treatment options used across global oncology systems. Surgery remains the primary intervention due to its role in tumor removal and survival improvement. Chemotherapy supports disease control through anthracycline-, ifosfamide-, gemcitabine-, and taxane-based regimens that guide systemic therapy plans. Radiation therapy strengthens local control through external beam methods and brachytherapy techniques. Hormone therapy serves selected hormone-receptor–positive cases where targeted hormonal pathways matter. Targeted therapy with agents such as pazopanib and trabectedin shapes growth in advanced disease lines. Immunotherapy gains interest through checkpoint inhibitors and novel agents under study. It creates momentum for next-generation systemic approaches.
- For instance, doxorubicin has demonstrated response rates of about 14–20% in advanced soft-tissue sarcoma across multiple trials.
End-User Segments
Hospitals lead treatment adoption due to strong surgical capacity and structured oncology teams. These centers manage complex cases and support integrated diagnostic workflows. Specialty and oncology clinics contribute through focused sarcoma programs with personalized treatment plans. Cancer research centers and academic institutes support innovation through trial enrollment and biomarker studies. Their involvement drives refinement of advanced therapeutic pathways and long-term survival research. Each end-user group strengthens clinical readiness with coordinated care models. The Uterine Leiomyosarcoma Market benefits from expanding infrastructure that enhances timely access to therapy. It supports continued growth in advanced care delivery.
- For example, MD Anderson Cancer Center conducts over 1,200 active clinical trials at any time, including multiple sarcoma studies.
Segmentation:
Treatment Segments
- Surgery (hysterectomy and related surgical interventions)
- Chemotherapy (anthracycline-, ifosfamide-, gemcitabine-, and taxane-based regimens)
- Radiation therapy (external beam and brachytherapy)
- Hormone therapy (for selected hormone-receptor–positive cases)
- Targeted therapy (pazopanib, trabectedin, and other kinase/targeted agents)
- Immunotherapy and emerging systemic therapies (checkpoint inhibitors and novel agents)
End-User Segments
- Hospitals
- Specialty / oncology clinics
- Cancer research centers and academic institutes
Regional Segments
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America Leadership and Strong Clinical Readiness
North America holds the largest share of the Uterine Leiomyosarcoma Market at nearly 40% due to advanced oncology networks and strong diagnostic capacity. The United States drives most activity through high adoption of targeted drugs and precision tools. Hospitals operate specialized sarcoma units that support rapid intervention and structured follow-up care. Drug makers introduce new therapies in this region first due to stronger regulatory clarity. Providers rely on robust imaging infrastructure that improves early detection patterns. The Uterine Leiomyosarcoma Market gains steady momentum here through high awareness and consistent treatment access. It remains the benchmark region for modern oncology practice.
Europe’s Expanding Access to Targeted and Systemic Therapies
Europe accounts for roughly 30% share with strong involvement from Germany, France, Italy, and the United Kingdom. Regional health systems support broad access to approved oncology drugs through structured reimbursement pathways. Hospitals adopt multidisciplinary models that guide therapy selection for complex sarcoma cases. Research networks contribute to trial activity that expands treatment confidence. Providers follow well-defined diagnostic protocols that reduce variation in care quality. Clinical teams integrate molecular testing into routine evaluation with rising frequency. The Uterine Leiomyosarcoma Market benefits from Europe’s balanced focus on precision medicine and universal healthcare models. It strengthens long-term adoption in advanced treatment categories.
Asia-Pacific, Latin America, and Middle East & Africa Growth Outlook
Asia-Pacific represents nearly 20% share and shows the fastest growth due to expanding cancer centers in China, Japan, India, and South Korea. Hospitals increase investment in imaging tools that improve tumor detection levels. Oncology clinics adopt modern systemic therapies as healthcare spending rises. Latin America holds close to 6% share with steady progress in Brazil and Mexico where diagnostic capacity improves each year. Middle East & Africa account for roughly 4% share due to limited infrastructure but rising investment in tertiary care. Each emerging region strengthens its clinical pathway with new oncology programs and improved workforce training. The Uterine Leiomyosarcoma Market gains broad visibility across these regions through steady expansion. It shows long-term potential as access to specialized treatment improves.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Pfizer Inc.
- Novartis AG
- Eli Lilly and Company
- Bristol-Myers Squibb Company
- Merck & Co., Inc.
- GlaxoSmithKline (GSK plc)
- Johnson & Johnson / Janssen
- AstraZeneca plc
- Bayer AG
- F. Hoffmann-La Roche Ltd / Genentech
- Amgen Inc.
- Sanofi
- Takeda Pharmaceutical Company Limited
- Eisai Co., Ltd.
Competitive Analysis:
The Uterine Leiomyosarcoma Market features strong competition among global oncology drug makers focused on targeted, immunotherapy, and chemotherapy advancements. Companies strengthen portfolios through pipeline expansion and broader clinical trial activity. It drives innovation across treatment classes that support tumor control in advanced cases. Firms invest in predictive biomarkers to sharpen therapy selection and improve response consistency. Strategic collaborations help organizations access new research centers and patient pools. Oncology leaders prioritize safety refinement and long-term survival data to secure regulatory approval. Market rivalry grows as hospitals and clinics demand therapies with stronger evidence. The landscape continues to shift through scientific progress and rising patient awareness.
Recent Developments:
- In October 2025, Eisai presented clinical research at ESMO Congress 2025 highlighting lenvatinib plus pembrolizumab combination for advanced endometrial carcinoma, featuring 5-year overall survival data from the Phase 3 Study 309/KEYNOTE-775 trial and additional 1-year follow-up results from the Phase 3 LEAP-001 study in first-line advanced or recurrent endometrial carcinoma.
- In April 2025, Merck KGaA announced an agreement to acquire SpringWorks Therapeutics for an enterprise value of $3.4 billion (approximately €3 billion). The acquisition was completed on June 30, 2025. SpringWorks’ portfolio includes Ogsiveo (nirogacestat) for desmoid tumors and Gomekli (mirdametinib) for neurofibromatosis type 1-associated plexiform neurofibromas.
- In January 2025, Eli Lilly announced its acquisition of Scorpion Therapeutics’ PI3Kα inhibitor program (ST-478) for up to $2.5 billion in cash, including upfront and contingent regulatory and sales milestone payments. ST-478 is currently in initial trials for breast cancer and other advanced solid tumors.
- In January 2025, GSK announced the acquisition of Idrx Inc. for $1 billion upfront to add to its cancer pipeline. In July 2024, the FDA expanded the approval of Jemperli (dostarlimab) plus chemotherapy to all adult patients with primary advanced or recurrent endometrial cancer, following positive Phase III data.
Report Coverage:
The research report offers an in-depth analysis based on Treatment Segments and End-User Segments. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Precision oncology adoption will accelerate treatment personalization across global centers.
- Genomic profiling use will expand as hospitals refine diagnostic pathways.
- Immunotherapy combinations will gain stronger evidence across complex disease cases.
- Targeted agents will enter broader use through advanced trial results.
- Hospitals will strengthen multidisciplinary sarcoma units for better treatment planning.
- Emerging markets will invest in oncology infrastructure to improve care access.
- Research collaboration networks will expand patient enrollment for rare cancer trials.
- New biomarkers will support early diagnosis and guide therapy response prediction.
- Digital health tools will improve treatment monitoring and long-term patient engagement.
- The Uterine Leiomyosarcoma Market will evolve with steady innovation in systemic therapies.




