Market Overview
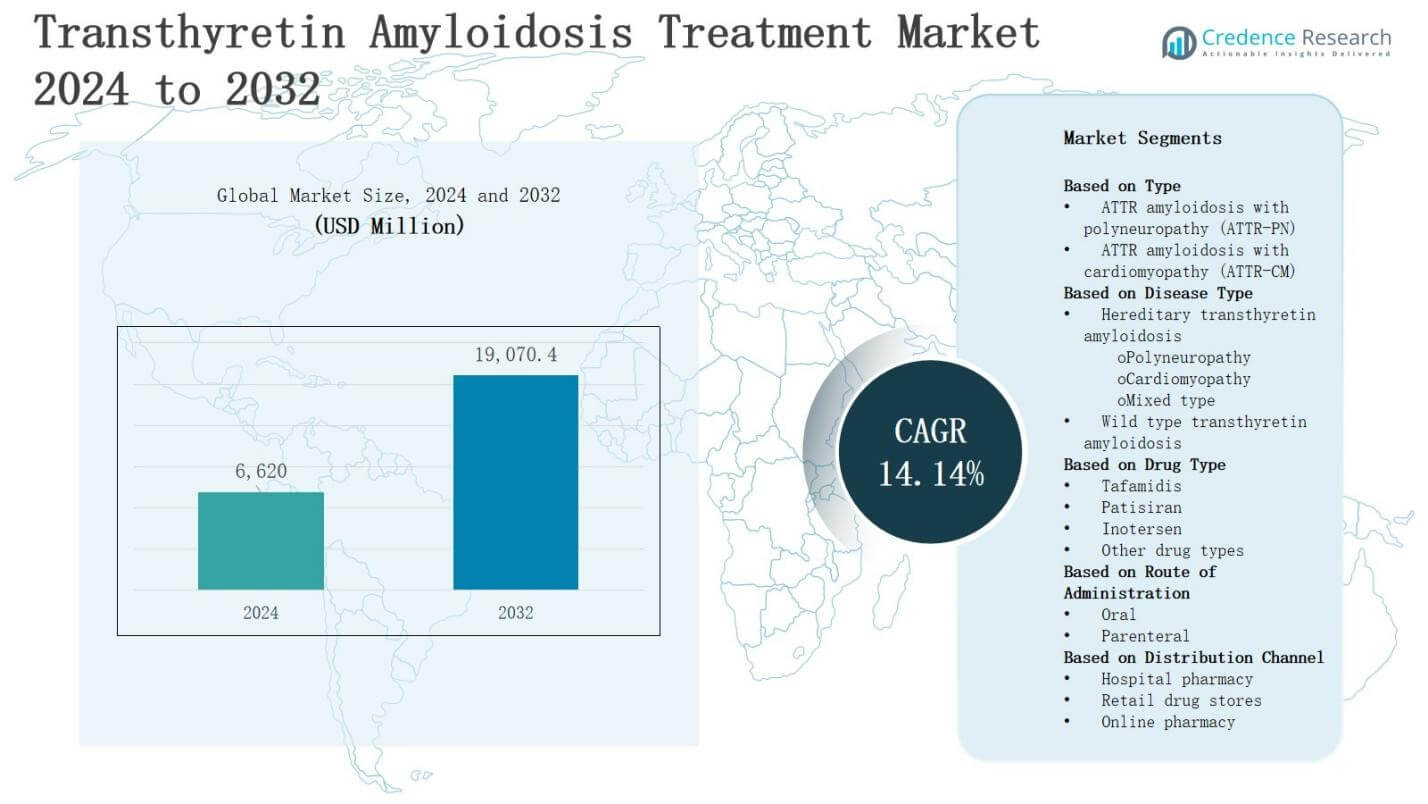
The transthyretin amyloidosis treatment market is projected to grow from USD 6,620 million in 2024 to USD 19,070.4 million by 2032, registering a strong CAGR of 14.14% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Transthyretin Amyloidosis Treatment Market Size 2024 |
USD 6,620 Million |
| Transthyretin Amyloidosis Treatment Market, CAGR |
14.14% |
| Transthyretin Amyloidosis Treatment Market Size 2032 |
USD 19,070.4 Million |
The transthyretin amyloidosis treatment market is driven by rising prevalence of hereditary and wild-type ATTR, increasing awareness among healthcare professionals, and advancements in genetic testing that enable early diagnosis. Strong demand for disease-modifying therapies, such as RNA interference and stabilizers, supports market expansion, while favorable regulatory approvals and orphan drug designations accelerate product launches. Key trends include growing adoption of gene therapies, expansion of clinical trials targeting broader patient populations, and strategic collaborations between biopharma companies to enhance treatment accessibility. Integration of digital health tools for patient monitoring further strengthens the market’s growth trajectory.
The transthyretin amyloidosis treatment market demonstrates strong geographical diversity, with North America leading growth, followed by Europe with robust healthcare systems and research collaborations. Asia Pacific shows rising adoption driven by Japan, China, and emerging economies, while Latin America progresses through Brazil and Mexico’s rare disease initiatives. The Middle East & Africa remains at an early stage, with stronger uptake in Gulf nations. Key players include Pfizer Inc., Alnylam Pharmaceuticals, Ionis Pharmaceuticals, BridgeBio Pharma, Bristol-Myers Squibb, AstraZeneca, Johnson & Johnson, Astellas Pharma, Prothena, Acrotech Biopharma, and SOM Innovation Biotech.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The transthyretin amyloidosis treatment market is projected to grow from USD 6,620 million in 2024 to USD 19,070.4 million by 2032, at a CAGR of 14.14%.
- Rising prevalence of hereditary and wild-type ATTR, coupled with improved genetic testing and biomarker identification, drives early diagnosis and treatment adoption.
- Strong pipeline of disease-modifying therapies, including RNA interference drugs, stabilizers, and gene-editing platforms, fuels innovation and long-term growth.
- High treatment costs and reimbursement challenges remain key barriers, limiting accessibility, especially in developing economies.
- North America leads with 45% share, followed by Europe at 28%, Asia Pacific at 18%, Latin America at 5%, and Middle East & Africa at 4%.
- Strategic collaborations between pharma companies, biotech startups, and research institutions accelerate clinical trials and expand treatment access.
- Key players include Pfizer, Alnylam Pharmaceuticals, Ionis Pharmaceuticals, BridgeBio Pharma, Bristol-Myers Squibb, AstraZeneca, Johnson & Johnson, Astellas Pharma, Prothena, Acrotech Biopharma, and SOM Innovation Biotech.
Market Drivers
Rising Prevalence and Improved Diagnosis Rates
The transthyretin amyloidosis treatment market benefits from the increasing prevalence of hereditary and wild-type ATTR across aging populations. It gains further momentum from advancements in diagnostic techniques, including genetic testing, imaging modalities, and biomarker identification. Physicians now identify patients at earlier stages, expanding treatment eligibility. Growing awareness among healthcare providers and patient advocacy organizations ensures better detection rates. Governments and health agencies also emphasize rare disease registries, reinforcing the need for specialized therapies and clinical interventions.
- For instance, AstraZeneca has introduced Eplontersen, expanding treatment options for this rare disease.
Advancements in Therapeutic Innovations
The transthyretin amyloidosis treatment market grows rapidly due to novel therapies such as TTR stabilizers, RNA interference drugs, and gene-editing platforms. It reflects strong investment in biotechnology, with biopharma companies pursuing pipeline expansion. Market players secure orphan drug designations, fast-track approvals, and breakthrough status, driving innovation. Patient-centric approaches push for more effective, less invasive therapies. Clinical trial expansions across global regions strengthen treatment availability. Rising R&D expenditure and technological integration continue shaping next-generation solutions.
- For instance, Pfizer’s tafamidis, a TTR stabilizer, demonstrated a 13% increase in survival at 30 months for patients with transthyretin amyloid cardiomyopathy in a pivotal phase 3 trial.
Regulatory Approvals and Incentive Programs
The transthyretin amyloidosis treatment market benefits significantly from favorable regulatory pathways supporting rare disease therapies. It experiences accelerated drug approvals, compassionate use programs, and pricing incentives. Regulatory bodies encourage innovation by providing tax credits, market exclusivity, and priority review vouchers. Governments recognize the burden of untreated ATTR, pushing policies that enable rapid adoption of advanced drugs. This supportive ecosystem attracts new entrants, accelerates commercialization, and strengthens investor confidence in long-term therapeutic development.
Strategic Collaborations and Expanding Access
The transthyretin amyloidosis treatment market gains momentum from collaborations among pharmaceutical firms, biotech startups, and academic institutions. It leverages partnerships to advance clinical research, co-develop therapies, and improve distribution networks. Companies pursue licensing deals and joint ventures to expand treatment reach in emerging economies. Rising focus on value-based care models strengthens affordability and reimbursement options. Advocacy groups drive awareness campaigns, encouraging earlier diagnosis and patient enrollment in trials. Such strategic initiatives sustain long-term industry growth.
Market Trends
Growing Shift Toward Gene and RNA-Based Therapies
The transthyretin amyloidosis treatment market is witnessing a strong trend toward gene therapy and RNA-based approaches. It reflects significant progress with RNA interference and antisense oligonucleotide drugs that reduce abnormal protein production at the source. Companies invest heavily in CRISPR and other advanced platforms to provide long-term or potentially curative outcomes. Expanding clinical trials targeting broader genetic subgroups highlights this trend. The market demonstrates rising confidence in precision medicine, positioning gene-targeted solutions as future standards.
- For instance, Alnylam Pharmaceuticals developed patisiran, an RNA interference (RNAi) drug that decreases transthyretin (TTR) liver production by about 80% for up to 12 weeks, effectively targeting the root cause of the disease.
Integration of Digital Health and Remote Monitoring Tools
The transthyretin amyloidosis treatment market adopts digital health technologies to improve patient management and monitoring outcomes. It increasingly incorporates wearable devices, AI-based analytics, and telehealth platforms to track disease progression and treatment adherence. These solutions allow real-time data collection, enabling physicians to adjust therapy more effectively. Patients benefit from remote access to specialists, particularly in underserved regions. Healthcare providers emphasize integrated care models, strengthening the role of digital tools in supporting long-term treatment strategies.
Expansion of Clinical Trials Across Global Regions
The transthyretin amyloidosis treatment market shows rapid expansion in global clinical trials aimed at addressing diverse populations. It highlights an increasing shift toward multi-regional studies that assess therapy effectiveness across genetic, demographic, and environmental variations. Pharmaceutical companies collaborate with international research organizations to accelerate development. Expanding patient enrollment improves trial diversity, ensuring robust clinical data. Growing trial activity in Asia and Latin America reinforces opportunities, broadening treatment accessibility while supporting long-term pipeline growth.
- For instance, Alnylam Pharmaceuticals’ clinical trials with patisiran (Onpattro) have demonstrated significant reductions in amyloid deposits and neuropathies, generating robust clinical data that supports early disease-modifying treatment effects.
Rising Focus on Combination Therapies and Personalized Medicine
The transthyretin amyloidosis treatment market reflects a clear trend toward combination therapies that optimize patient outcomes. It includes approaches that pair stabilizers with RNA-based drugs or gene therapies to slow progression and improve quality of life. Personalized medicine advances guide treatment selection based on genetic profiles and disease stage. Physicians increasingly tailor regimens to specific patient needs, improving efficacy. Pharmaceutical companies prioritize biomarker-driven development, solidifying personalized solutions as essential in future clinical practice.
Market Challenges Analysis
High Treatment Costs and Limited Accessibility
The transthyretin amyloidosis treatment market faces significant challenges due to the high cost of therapies, which limits accessibility for many patients. It includes advanced drugs such as RNA-based therapies and stabilizers that are priced at premium levels, straining healthcare budgets. Reimbursement barriers across different regions further complicate patient access. Developing economies experience the greatest hurdles due to limited healthcare infrastructure and financial constraints. The need for cost-effective alternatives remains urgent to ensure equitable treatment availability worldwide.
Complex Diagnosis and Limited Awareness
The transthyretin amyloidosis treatment market also struggles with delayed diagnosis and limited disease awareness among healthcare providers. It suffers from misdiagnosis because symptoms often overlap with more common conditions such as cardiomyopathy or neuropathy. Lack of standardized screening protocols delays intervention and reduces therapy effectiveness. Patient awareness campaigns remain insufficient in many regions, leaving cases undetected until advanced stages. Addressing diagnostic complexity and improving education for both physicians and patients is essential to strengthen treatment outcome
Market Opportunities
Emerging Role of Gene Therapy and Novel Drug Classes
The transthyretin amyloidosis treatment market presents strong opportunities through the rapid development of gene therapies and novel drug classes. It benefits from expanding research in RNA interference, antisense technologies, and CRISPR-based editing, which aim to address the root cause of the disease. Pharmaceutical companies are investing in pipeline diversification to deliver long-term or potentially curative outcomes. Increasing regulatory support for breakthrough therapies enhances market entry. Growing patient enrollment in global trials strengthens clinical validation, reinforcing the commercial potential of innovative solutions.
Expansion Across Untapped Regions and Collaborative Models
The transthyretin amyloidosis treatment market creates opportunities by expanding into untapped regions with growing healthcare infrastructure. It gains traction from rising investments in Latin America, Asia Pacific, and the Middle East, where diagnosis rates and awareness are steadily improving. Strategic collaborations between global pharmaceutical firms, biotech startups, and healthcare providers enhance treatment accessibility. Governments support rare disease programs, creating reimbursement pathways that improve adoption. Partnerships with advocacy organizations also broaden education efforts, increasing patient engagement and expanding therapeutic reach worldwide.
Market Segmentation Analysis:
By Type
The transthyretin amyloidosis treatment market is segmented into ATTR amyloidosis with polyneuropathy (ATTR-PN) and ATTR amyloidosis with cardiomyopathy (ATTR-CM). It shows strong demand for ATTR-CM therapies due to rising incidence in aging populations and growing recognition of cardiac involvement. ATTR-PN remains significant, supported by RNA-based treatments that effectively target neuropathic symptoms. Increasing clinical trial activity in both segments enhances therapeutic options. Expanding adoption of disease-modifying drugs drives growth across both categories, reinforcing their role in long-term disease management.
- For instance, Pfizer’s drug Vyndaqel (tafamidis) gained global approval for ATTR-CM after its ATTR-ACT trial demonstrated a 31% reduction in all-cause mortality and nearly halved hospitalizations for cardiovascular events in patients.
By Disease Type
The market divides into hereditary transthyretin amyloidosis and wild-type transthyretin amyloidosis. It reflects increasing identification of hereditary cases, with subtypes including polyneuropathy, cardiomyopathy, and mixed forms. Advances in genetic testing boost early detection, particularly for hereditary polyneuropathy. Wild-type ATTR represents a growing share, driven by rising prevalence among elderly populations. Improved awareness and diagnostic capabilities broaden patient pools. Both categories benefit from precision therapies, reinforcing treatment adoption and expanding the global footprint of available interventions.
- For instance, Pfizer offers tafamidis (VYNDAQEL/VYNDAMAX), approved since 2019, which stabilizes transthyretin to slow disease progression mainly in cardiomyopathy cases.
By Drug Type
The market covers tafamidis, patisiran, inotersen, and other drug types. It highlights tafamidis as a leading option for cardiomyopathy, supported by proven efficacy and widespread regulatory approvals. Patisiran and inotersen gain traction in addressing hereditary polyneuropathy, expanding their clinical relevance. Emerging therapies in the pipeline strengthen the competitive landscape by offering new mechanisms of action. Combination approaches and second-generation RNA therapies create additional opportunities, positioning drug innovation as a core growth driver across this therapeutic area.
Segments:
Based on Type
- ATTR amyloidosis with polyneuropathy (ATTR-PN)
- ATTR amyloidosis with cardiomyopathy (ATTR-CM)
Based on Disease Type
- Hereditary transthyretin amyloidosis
-
- Polyneuropathy
- Cardiomyopathy
- Mixed type
- Wild type transthyretin amyloidosis
Based on Drug Type
- Tafamidis
- Patisiran
- Inotersen
- Other drug types
Based on Route of Administration
Based on Distribution Channel
- Hospital pharmacy
- Retail drug stores
- Online pharmacy
Based on the Geography:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis
North America
North America dominates the transthyretin amyloidosis treatment market with 45% share, driven by advanced healthcare infrastructure and strong adoption of innovative therapies. It benefits from early regulatory approvals, favorable reimbursement policies, and high disease awareness among physicians. Leading pharmaceutical companies operate extensive clinical programs in the United States, ensuring rapid product availability. Canada contributes with improved access through rare disease initiatives. High diagnostic rates and patient advocacy efforts strengthen treatment uptake. Continuous investment in R&D supports long-term growth momentum across this region.
Europe
Europe holds 28% share of the transthyretin amyloidosis treatment market, supported by rising focus on rare disease management and regulatory frameworks that encourage orphan drug development. It benefits from robust healthcare systems in countries such as Germany, France, and the United Kingdom, which facilitate early diagnosis and adoption. Increasing collaborations between academic research centers and pharmaceutical firms enhance clinical trial activity. Governments emphasize rare disease registries to track patient outcomes. Broader access to advanced genetic testing strengthens identification rates, supporting demand for new therapies.
Asia Pacific
Asia Pacific accounts for 18% share of the transthyretin amyloidosis treatment market, driven by growing healthcare expenditure and improving diagnostic capabilities. It experiences rising prevalence of both hereditary and wild-type ATTR, particularly in Japan and China. Governments invest in genetic testing infrastructure, enhancing early disease recognition. Pharmaceutical companies expand partnerships with local firms to accelerate clinical trial enrollment and therapy distribution. Growing awareness campaigns and specialist training programs encourage wider adoption. Emerging markets in Southeast Asia present untapped opportunities for expansion.
Latin America
Latin America represents 5% share of the transthyretin amyloidosis treatment market, supported by gradual improvements in healthcare systems and patient awareness. It faces challenges from limited diagnostic infrastructure and cost-related access barriers. Brazil and Mexico lead adoption through specialized treatment centers and government initiatives for rare diseases. Multinational companies collaborate with local healthcare providers to strengthen supply chains. Awareness campaigns expand patient identification rates. Rising interest in clinical research further supports the region’s potential growth trajectory.
Middle East & Africa
The Middle East & Africa holds 4% share of the transthyretin amyloidosis treatment market, reflecting early-stage adoption of advanced therapies. It is characterized by disparities in healthcare access, with higher adoption in Gulf countries compared to Sub-Saharan regions. Governments implement rare disease policies to improve patient access, particularly in Saudi Arabia and the UAE. Multinational pharmaceutical companies enter through strategic partnerships with regional distributors. Awareness programs led by advocacy groups are expanding. Rising healthcare investments provide long-term growth opportunities.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Pfizer Inc.
- BridgeBio Pharma, Inc.
- Prothena Biosciences Limited
- Johnson & Johnson
- Alnylam Pharmaceuticals, Inc.
- SOM Innovation Biotech, S.L.
- Bristol-Myers Squibb Company
- AstraZeneca PLC
- Acrotech Biopharma, LLC
- Astellas Pharma Inc.
- Ionis Pharmaceuticals, Inc.
Competitive Analysis
The transthyretin amyloidosis treatment market is highly competitive with established pharmaceutical leaders and emerging biotech firms driving innovation across multiple therapy classes. It features Pfizer Inc. as a key player with tafamidis, the first widely approved therapy for cardiomyopathy, while Alnylam Pharmaceuticals, Inc. leads RNA interference treatments with patisiran. Ionis Pharmaceuticals, Inc. strengthens the market with inotersen, targeting hereditary polyneuropathy. BridgeBio Pharma, Inc. and Prothena Biosciences Limited focus on advancing next-generation stabilizers and novel mechanisms to expand patient coverage. Bristol-Myers Squibb Company and AstraZeneca PLC emphasize pipeline diversification through research collaborations, while Johnson & Johnson leverages broad resources to accelerate late-stage developments. Astellas Pharma Inc. and Acrotech Biopharma, LLC pursue strategic trials to strengthen regional presence. SOM Innovation Biotech, S.L. contributes with niche research in disease-modifying approaches. It demonstrates intense R&D activity, strong reliance on orphan drug designations, and strategic alliances to ensure global market expansion. The competitive landscape continues to evolve with partnerships, clinical trial advancements, and increasing emphasis on personalized and combination therapies.
Recent Developments
- In June 2025, Alnylam Pharmaceuticals received European Commission approval for AMVUTTRA® (vutrisiran) to treat transthyretin amyloidosis with cardiomyopathy (ATTR-CM) in adults, marking the first RNA interference therapy authorized for both ATTR-CM and hereditary ATTR polyneuropathy.
- In April 2025, BridgeBio Pharma secured approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for BEYONTTRA® (acoramidis) to treat wild-type and hereditary ATTR amyloidosis with cardiomyopathy, highlighting its near-complete (≥90%) transthyretin stabilization capability.
- In October 2024, AstraZeneca and Ionis Pharmaceuticals’ drug Wainua (eplontersen) received a positive EU CHMP approval recommendation for hereditary transthyretin amyloidosis with polyneuropathy (hATTR-PN).
- In January 2024, Wainua (eplontersen) was launched in the US for treating hereditary transthyretin amyloidosis.
Market Concentration & Characteristics
The transthyretin amyloidosis treatment market exhibits a moderately high concentration, with a few global pharmaceutical leaders and specialized biotech firms dominating product innovation and commercialization. It is shaped by strong reliance on orphan drug designations, fast-track regulatory approvals, and exclusivity incentives that allow established players to maintain competitive advantage. Companies such as Pfizer, Alnylam Pharmaceuticals, and Ionis Pharmaceuticals hold significant positions through disease-modifying therapies, while emerging firms like BridgeBio Pharma and Prothena expand the pipeline with next-generation stabilizers and gene-targeted approaches. The market demonstrates characteristics of high R&D intensity, strategic collaborations, and global clinical trial expansion. It faces cost-related access barriers, yet continues to attract investment due to unmet medical needs and growing prevalence of hereditary and wild-type ATTR. Competitive dynamics reflect a balance between large firms leveraging extensive resources and smaller innovators focusing on breakthrough technologies, ensuring sustained growth and continuous evolution of treatment standards.
Report Coverage
The research report offers an in-depth analysis based on Type, Disease Type, Drug Type, Route of Administartion, Distribution Channel and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will witness wider adoption of gene and RNA-based therapies offering long-term disease control.
- Expansion of global clinical trials will strengthen data diversity and accelerate regulatory approvals.
- Broader access to genetic testing will support earlier diagnosis and expand eligible patient populations.
- Combination therapies will gain importance to enhance treatment efficacy and improve patient outcomes.
- Strategic collaborations between pharma companies and biotech firms will drive faster innovation.
- Digital health integration will improve patient monitoring, adherence, and long-term care management.
- Emerging markets in Asia Pacific, Latin America, and the Middle East will provide new growth opportunities.
- Healthcare policies and reimbursement frameworks will evolve to improve treatment affordability.
- Competition will intensify as new entrants and pipeline products challenge existing therapies.
- Patient advocacy initiatives will continue to raise awareness and improve diagnosis rates worldwide.




