Market Overview:
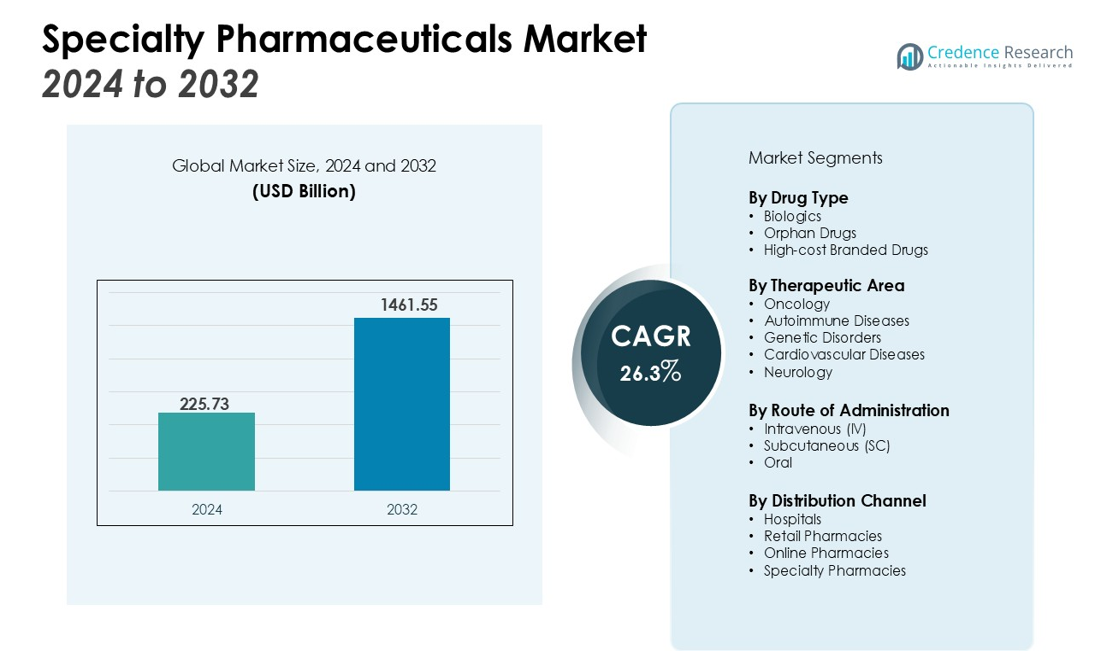
The Specialty Pharmaceuticals Market size was valued at USD 225.73 billion in 2024 and is anticipated to reach USD 1461.55 billion by 2032, at a CAGR of 26.3% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Specialty Pharmaceuticals Market Size 2024 |
USD 225.73 billion |
| Specialty Pharmaceuticals Market, CAGR |
26.3% |
| Specialty Pharmaceuticals Market Size 2032 |
USD 1461.55 billion |
Key drivers of market growth include rising healthcare expenditures, improved healthcare access in emerging markets, and the growing adoption of biologics for the treatment of cancer, autoimmune disorders, and genetic diseases. Additionally, the increasing investment in R&D by pharmaceutical companies to develop targeted therapies and personalized treatment options is expected to significantly contribute to market expansion. The need for specialized treatments to manage rare diseases is also propelling the market forward.
Regionally, North America holds the largest market share, driven by a robust healthcare infrastructure, high healthcare spending, and advanced research in pharmaceuticals. Europe follows closely, with significant growth driven by the growing healthcare needs in the aging population. Meanwhile, the Asia Pacific region is anticipated to witness the fastest growth, fueled by expanding healthcare access, rising disposable incomes, and the increasing burden of chronic diseases.
Market Insights:
- The specialty pharmaceuticals market was valued at USD 225.73 billion in 2024 and is projected to reach USD 1461.55 billion by 2032, growing at a CAGR of 26.3% during the forecast period.
- Increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular conditions drives the demand for specialty drugs. These diseases require specialized treatments, including biologics and orphan drugs, which significantly contribute to market growth.
- Advances in biotechnology and personalized medicine are accelerating market growth. Targeted therapies based on genomics and gene therapy offer more effective and individualized treatments, expanding the market for specialty pharmaceuticals.
- Rising healthcare expenditures, especially in emerging markets, are improving access to specialty pharmaceuticals. With growing investments in healthcare infrastructure, demand for high-cost treatments like biologics and orphan drugs is increasing.
- Supportive regulatory frameworks, such as faster approval processes and market exclusivity incentives, have encouraged pharmaceutical companies to develop specialty drugs. This regulatory support speeds up the availability of innovative treatments for patients in need.
- The high cost of specialty pharmaceuticals remains a significant challenge. The expense of biologics and orphan drugs can limit access to treatments, especially in emerging markets, impacting their widespread adoption.
- North America dominates the global market with a 40% share, driven by advanced healthcare infrastructure and strong research initiatives. Europe holds 30%, fueled by an aging population and increasing access to specialty treatments, while Asia-Pacific is growing rapidly, expected to reach 20% by 2032.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Increasing Prevalence of Chronic Diseases
The rise in chronic diseases such as cancer, diabetes, and cardiovascular conditions is a primary driver for the growth of the specialty pharmaceuticals market. The demand for innovative treatments to manage these conditions is increasing as the global population ages. These diseases often require specialized therapies that can be provided through biologics and orphan drugs, which are a significant portion of the specialty pharmaceuticals market. The ability of these treatments to provide better outcomes in managing chronic illnesses enhances their adoption across healthcare systems worldwide.
- For instance, to address the increasing need for treatments for uncommon chronic conditions, the FDA had granted 6,340 orphan drug designations by the end of 2022, providing development incentives for therapies across 1,079 rare diseases.
Advancements in Biotechnology and Personalized Medicine
Technological advancements in biotechnology have propelled the development of new and more effective specialty pharmaceuticals. Breakthroughs in genomics, gene therapy, and molecular biology enable the creation of targeted therapies that address the specific needs of individual patients. This shift towards personalized medicine has been a significant driver, as therapies are tailored to genetic profiles, ensuring more effective treatments and fewer side effects. Such innovations expand the specialty pharmaceuticals market, offering new avenues for addressing complex health issues.
- For instance, the FDA’s approval of Bluebird Bio’s gene therapy, Lyfgenia, for Sickle Cell Disease was supported by efficacy data from 36 patients in its HGB-206 clinical trial.
Rising Healthcare Expenditures and Increased Access to Treatments
Healthcare spending is increasing globally, particularly in emerging markets, where healthcare infrastructure is improving rapidly. As governments and private sectors invest in healthcare systems, access to specialty pharmaceuticals improves, creating a growing demand for these high-cost treatments. With a focus on treating rare and complex diseases, specialty pharmaceuticals play a crucial role in expanding the treatment options available to patients. Enhanced reimbursement policies also contribute to the market’s growth, ensuring better patient access to specialty treatments.
Supportive Regulatory Environment for Drug Development
Regulatory agencies, including the FDA and EMA, have streamlined processes for the approval of specialty pharmaceuticals, particularly for orphan drugs. The provision of incentives such as extended market exclusivity and fast-tracked approval processes has encouraged pharmaceutical companies to invest in rare disease treatments. This regulatory support accelerates the development and availability of specialty pharmaceuticals, ensuring more patients benefit from cutting-edge treatments. With fewer barriers to market entry for specialized drugs, the overall growth of the sector is strengthened.
Market Trends:
Shift Towards Personalized Medicine and Targeted Therapies
One of the prominent trends in the specialty pharmaceuticals market is the growing shift toward personalized medicine. Advances in biotechnology and genomics are enabling the development of targeted therapies that focus on the unique genetic makeup of individuals. These treatments are increasingly being used for conditions such as cancer, autoimmune diseases, and genetic disorders, ensuring more effective and less toxic therapies. Specialty pharmaceuticals play a crucial role in this transformation, offering therapies that are tailored to specific patient profiles. As precision medicine becomes more prevalent, pharmaceutical companies are investing heavily in R&D to create drugs that meet the needs of diverse patient populations. This trend is expected to continue driving growth in the specialty pharmaceuticals market as the demand for personalized treatment solutions rises.
- For instance, Ginkgo Bioworks is enhancing the manufacturing processes vital for personalized medicine, having been awarded a 4-year contract from DARPA to create a more efficient method for producing complex therapeutic proteins.
Increasing Adoption of Biologics and Orphan Drugs
Biologics and orphan drugs are becoming increasingly integral to the specialty pharmaceuticals market. These drugs, often used to treat rare or complex diseases, provide advanced therapeutic options that were previously unavailable. The growing adoption of biologics, in particular, has reshaped the landscape of chronic disease management, offering more targeted and effective treatments. As healthcare systems around the world aim to improve patient outcomes, the demand for orphan drugs is also rising, driven by the need for therapies addressing unmet medical needs. The market for these specialized drugs continues to expand as pharmaceutical companies focus on the development of novel biologics and orphan drugs, fueling innovation in the industry. This trend reflects the shift towards more sophisticated and individualized approaches to healthcare, ultimately driving the specialty pharmaceuticals market forward.
- For instance, Regeneron Pharmaceuticals achieved a significant breakthrough with its biologic, pozelimab, for treating the ultra-rare CHAPLE disease.
Market Challenges Analysis:
High Costs of Specialty Pharmaceuticals and Affordability Issues
One of the major challenges faced by the specialty pharmaceuticals market is the high cost of treatments. The development and manufacturing of specialty drugs, particularly biologics and orphan drugs, are expensive due to complex production processes and the need for specialized research. These high costs often result in limited access for patients, especially in emerging markets with weaker healthcare systems. Health insurance providers and government programs struggle to accommodate the expense, leading to affordability challenges for patients. The price of these drugs puts pressure on both healthcare providers and patients, impacting the widespread adoption of specialty pharmaceuticals. Pharmaceutical companies must find ways to balance innovation with affordability to meet growing demand.
Regulatory Hurdles and Delays in Drug Approvals
The specialty pharmaceuticals market also faces challenges related to regulatory processes. Although regulatory bodies like the FDA and EMA have made efforts to streamline approval processes, delays still occur, particularly for complex biologics and orphan drugs. These delays hinder the timely availability of new treatments to patients in need. Furthermore, the stringent regulatory requirements can increase the time and cost required for drug development. These challenges create roadblocks for pharmaceutical companies, making it difficult to keep pace with market demand for specialized therapies. Navigating these regulatory complexities remains a significant hurdle in the growth of the specialty pharmaceuticals market.
Market Opportunities:
Expanding Access to Emerging Markets
One of the key opportunities in the specialty pharmaceuticals market lies in the expansion of access to emerging markets. With rapid economic growth and improving healthcare infrastructure, regions such as Asia-Pacific, Latin America, and the Middle East are experiencing increased demand for advanced medical treatments. These markets offer substantial growth potential as governments and private sectors focus on expanding healthcare coverage and providing better access to specialty drugs. Pharmaceutical companies have the chance to capitalize on these emerging opportunities by tailoring their product offerings to meet the specific needs of these regions. As healthcare systems continue to develop, specialty pharmaceuticals can provide essential solutions for treating complex and rare diseases, fostering market expansion.
Innovation in Drug Development and Personalized Medicine
Innovation in drug development, particularly in personalized medicine and biologics, presents another significant opportunity for the specialty pharmaceuticals market. Advancements in genomics, biotechnology, and artificial intelligence are enabling the creation of more targeted and effective therapies for a wide range of diseases. These innovations open new avenues for pharmaceutical companies to introduce novel treatments that address unmet medical needs. By focusing on personalized and precision medicine, companies can cater to growing demand for therapies that provide tailored treatment options, improving patient outcomes and enhancing market opportunities. This trend continues to fuel growth within the specialty pharmaceuticals sector, driving innovation and competition.
Market Segmentation Analysis:
By Drug Type
The specialty pharmaceuticals market is primarily segmented by drug type into biologics, orphan drugs, and high-cost branded drugs. Biologics lead the market, driven by their ability to target complex diseases with high precision, especially in oncology, immunology, and neurology. Orphan drugs also represent a significant segment, addressing rare diseases with unmet medical needs. These therapies often receive regulatory incentives, such as fast-track approvals and market exclusivity. High-cost branded drugs are another key segment, typically used for chronic diseases, and their market share is growing as treatment options for complex conditions expand.
- For instance, Amgen achieved a key clinical milestone with its biosimilar, ABP 501, in a study for moderate to severe rheumatoid arthritis, the trial successfully demonstrated efficacy, with 194 patients treated with ABP 501 meeting the primary ACR20 response endpoint at the 24-week mark.
By Therapeutic Area
The specialty pharmaceuticals market is dominated by oncology, autoimmune diseases, and genetic disorders. Oncology treatments are the largest therapeutic area, driven by the increasing incidence of cancer globally and the rise of personalized cancer therapies. Autoimmune diseases, including rheumatoid arthritis and multiple sclerosis, also see substantial growth, fueled by biologics and targeted therapies. The treatment of genetic disorders, such as rare inherited diseases, is growing due to advancements in gene therapies and orphan drug development.
By Route of Administration
The market for specialty pharmaceuticals is segmented by route of administration into intravenous (IV), subcutaneous (SC), and oral forms. IV administration holds a significant share, particularly for biologics, due to its direct and fast delivery of high-potency drugs. Subcutaneous injections are gaining popularity for chronic disease management, offering patients a more convenient option compared to IV therapies. Oral formulations are expanding as the demand for patient-friendly treatments increases, with several oral biologics emerging as alternatives to injectable therapies.
- For instance, Johnson & Johnson highlights the durability of its intravenous therapy, nipocalimab, in treating generalized myasthenia gravis (gMG), the company’s clinical program has successfully gathered follow-up data equivalent to 180 patient-years, reinforcing the therapy’s consistent long-term safety profile.
Segmentations:
By Drug Type
- Biologics
- Orphan Drugs
- High-cost Branded Drugs
By Therapeutic Area
- Oncology
- Autoimmune Diseases
- Genetic Disorders
- Cardiovascular Diseases
- Neurology
By Route of Administration
- Intravenous (IV)
- Subcutaneous (SC)
- Oral
By Distribution Channel
- Hospitals
- Retail Pharmacies
- Online Pharmacies
- Specialty Pharmacies
By Region
- North America
- Europe
- Germany
- France
- Italy
- U.K.
- Russia
- Rest of Europe
- Asia-Pacific
- India
- China
- Japan
- Rest of Asia-Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East and Africa
- GCC Countries
- South Africa
- Rest of Middle East and Africa
Regional Analysis:
North America: Dominance Driven by Healthcare Infrastructure and Innovation
North America holds the largest share of the global specialty pharmaceuticals market, accounting for 40%. This dominance is driven by advanced healthcare infrastructure, high healthcare expenditure, and a strong focus on medical research and innovation. The United States leads in the adoption of biologics, orphan drugs, and targeted therapies due to the increasing prevalence of chronic diseases and a growing aging population. Regulatory support from agencies like the FDA accelerates drug approvals, enabling quicker access to innovative treatments. North America’s well-established healthcare systems, along with high reimbursement rates, ensure strong market growth for specialty pharmaceuticals.
Europe: Growth Fueled by Aging Population and Expanding Healthcare Access
Europe holds a 30% share of the global specialty pharmaceuticals market, supported by an aging population and expanding access to advanced healthcare. The region’s robust regulatory framework and strong research initiatives support the growth of specialty drugs, particularly in cancer and rare disease treatments. Countries like Germany, the UK, and France are leading in the adoption of biologics and personalized medicine. Increased government spending on healthcare infrastructure and patient care enhances access to specialty pharmaceuticals, fueling market demand. With a focus on precision medicine and biologics, Europe presents a promising landscape for market expansion.
Asia-Pacific: Rapid Growth and Increasing Demand for Specialty Treatments
Asia-Pacific holds a 20% share of the global specialty pharmaceuticals market and is expected to experience the fastest growth. Rising healthcare investments and increasing awareness of specialized treatments contribute to this growth. Countries like China, India, and Japan are improving healthcare infrastructure and seeing greater demand for treatments targeting chronic diseases. The shift towards biologics and orphan drugs in these markets reflects the need for innovative therapies to address a rising burden of diseases. Government initiatives to improve healthcare access and investments in biotechnology will further boost the demand for specialty pharmaceuticals in the region.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Incyte
- Kamada
- AbbVie
- Amgen
- Merck & Co.
- Biocon Biologics
- Eli Lilly
- Genentech
- Knight Therapeutics
- Novartis
- GlaxoSmithKline
- Pfizer
- TAIHO PHARMACEUTICAL
Competitive Analysis:
The specialty pharmaceuticals market is highly competitive, with numerous key players focusing on biologics, orphan drugs, and targeted therapies. Major companies such as Novartis AG, Roche, and Johnson & Johnson are leading the market by continually expanding their product portfolios through innovative drug development. These companies are investing heavily in research and development to create specialized treatments for chronic diseases, cancer, and rare conditions. The market also sees increasing competition from emerging biotech firms that are introducing novel therapies, particularly in personalized medicine. Regulatory support, including faster approval processes and incentives for rare disease treatments, further intensifies competition. Market leaders are striving to gain a competitive edge through strategic partnerships, mergers, and acquisitions to strengthen their R&D capabilities and market presence. With high development costs, companies must balance innovation with affordability to maintain their market position in the evolving specialty pharmaceuticals landscape.
Recent Developments:
- In September 2025, AbbVie launched “The One & Only” campaign for BOTOX® Cosmetic, which shares the stories of real individuals.
- In June 2025, Biocon Biologics signed a Memorandum of Understanding with the National Cancer Society of Malaysia to improve patient access to affordable oncology biosimilars.
Report Coverage:
The research report offers an in-depth analysis based on Drug Type, Therapeutic Area, Route of Administration, Distribution Channel and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Continued advancements in biotechnology will lead to the development of more targeted and effective therapies for complex diseases.
- The growing focus on personalized medicine will increase the demand for specialized treatments tailored to individual genetic profiles.
- Expanding access to healthcare in emerging markets will drive higher adoption of specialty pharmaceuticals.
- Increased government and private sector investments in healthcare infrastructure will create more opportunities for market growth.
- The rising prevalence of chronic diseases, such as cancer, diabetes, and cardiovascular conditions, will continue to fuel demand for specialty pharmaceuticals.
- The expansion of biologics and gene therapies will play a significant role in addressing previously untreatable or rare diseases.
- Strategic collaborations and partnerships between pharmaceutical companies and biotech firms will lead to faster development and market introduction of new treatments.
- Regulatory agencies will maintain support for orphan drugs and other specialty treatments, ensuring quicker approvals and better market access.
- The rise of digital health technologies will enable better monitoring of patients and more effective management of chronic conditions through specialty drugs.
- Ongoing research in the field of genetic and stem cell therapies will unlock new treatment options for genetic disorders, further enhancing the specialty pharmaceuticals market.




