Market Overview
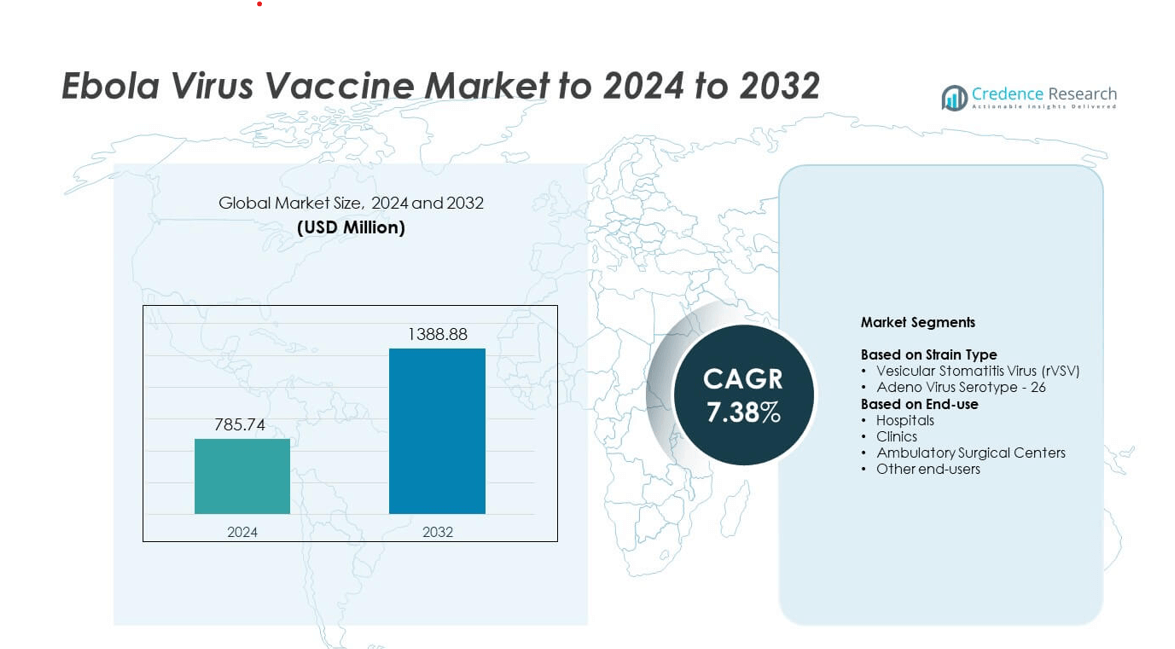
Ebola Virus Vaccine Market size was valued at USD 785.74 Million in 2024 and is anticipated to reach USD 1388.88 Million by 2032, at a CAGR of 7.38% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Ebola Virus Vaccine MarketSize 2024 |
USD 785.74 Million |
| Ebola Virus Vaccine Market, CAGR |
7.38% |
| Ebola Virus Vaccine Market Size 2032 |
USD 1388.88 Million |
The Ebola Virus Vaccine Market is shaped by leading companies such as Inovio Pharmaceuticals, Inc., Johnson & Johnson, GeoVax Labs, Inc., Mapp Biopharmaceutical, Inc., BioCryst Pharmaceuticals, Inc., GlaxoSmithKline plc, Merck & Co., Inc., Emergent BioSolutions Inc., Immunovaccine Inc. IMV Inc., and Bavarian Nordic A/S. These manufacturers compete through advanced viral-vector platforms, expanded clinical trials and strategic partnerships with global health agencies. North America leads the market with about 37 % share due to strong biopharma capacity, government-funded preparedness programs and rapid vaccine-stockpiling systems. Europe follows as a key region, supported by coordinated procurement and robust regulatory oversight.
Market Insights
- The Ebola Virus Vaccine Market was valued at USD 785.74 Million in 2024 and is projected to reach USD 1388.88 Million by 2032, growing at a CAGR of 7.38%.
- Strong demand grows as governments expand outbreak-preparedness budgets and prioritize rapid response, with the rVSV strain holding about 61 % share due to fast immunogenic protection.
- Trends include rising adoption of single-dose platforms, investment in multivalent vaccine research and improved cold-chain systems supporting wider access in remote regions.
- Competition strengthens as major manufacturers advance viral-vector technology, expand clinical trials and secure long-term supply agreements with global health networks.
- North America leads with nearly 37 % share due to strong biopharma infrastructure, while hospitals dominate end-use with about 48 % share; Europe and Asia Pacific follow with steady growth supported by preparedness programs and expanded vaccination capacity.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Segmentation Analysis:
By Strain Type
The vesicular stomatitis virus rVSV strain leads this segment with about 61% share in 2024 due to strong immunogenic response and rapid protection in high-risk exposure settings. Health agencies favor rVSV-based vaccines because the vector supports single-dose delivery and fast outbreak deployment. Adoption rises as global stockpiles expand and regulatory bodies approve wider emergency use. Adenovirus serotype 26 grows at a steady pace, supported by multi-dose stability and improved cold-chain flexibility in remote regions. Demand strengthens as countries invest in diversified vaccine platforms.
- For instance, Merck reported that by March 2023 it had supplied over 500,000 ERVEBO doses to the International Coordinating Group Ebola vaccine stockpile.
By End-use
Hospitals dominate this segment with nearly 48% share in 2024 driven by strong patient inflow, advanced vaccination units, and priority allocation during outbreak clusters. Hospitals receive most emergency vaccine supplies, which accelerates uptake and broadens immunization coverage among frontline workers. Clinics show growing adoption due to expansion of community vaccination programs, while ambulatory surgical centers participate in targeted immunization drives. Other end users, including NGOs and mobile health units, support coverage in rural and cross-border areas as governments scale rapid response capacity.
- For instance, Records for the year 2022 show that a total of 13,870 doses of the rVSV-ZEBOV Ebola vaccine were shipped from the global International Coordinating Group (ICG) stockpile for outbreak response and preventive vaccination activities globally.
Key Growth Drivers
Rising Outbreak Preparedness Investments
Global health authorities expand preparedness budgets to strengthen early response systems, which increases demand for Ebola vaccines. Governments and international organizations accelerate stockpiling to address recurring outbreaks in Central and West Africa. Expanded funding supports rapid procurement, wider immunization coverage, and broader emergency deployment capacity. Growing emphasis on cross-border coordination and faster containment strategies continues to push vaccine manufacturing and regulatory approvals. This rising focus on preventive readiness stands as a key growth driver in the Ebola Virus Vaccine Market.
- For instance, the Centers for Disease Control and Prevention (CDC) reported on the global Ebola vaccine stockpile in a 2024 report that included data from 2023. This report stated that as of December 2023, the stockpile held 518,890 doses of the rVSV-ZEBOV vaccine.
Advancements in Vaccine Platforms
Improved viral-vector technologies enhance immune response durability and shorten dosing schedules, increasing their acceptance in high-risk regions. Faster development cycles and stronger safety data support broader clinical adoption across endemic zones. Manufacturers deploy scalable production systems that deliver stable supplies for mass immunization programs. These advancements help reduce transmission risk and improve outbreak control timelines. This steady improvement in vaccine performance acts as a key growth driver for the Ebola Virus Vaccine Market.
- For instance, Janssen-supported research and analysis of pooled clinical trial data, including the Phase II trial NCT02564523, indicated that the Ad26-ZEBOV, MVA-BN-Filo vaccine regimen induced robust immune responses that were sustained for at least one year, with modeling suggesting persistence for up to 730 days (two years) and beyond
Increased International Health Partnerships
Collaborations between global organizations, vaccine developers, and regional health ministries expand outreach and accelerate distribution across remote zones. Joint initiatives improve surveillance, cold-chain support, and clinical training, making vaccination campaigns more effective. Multinational alliances strengthen donor funding, allowing low-income nations to access reliable doses. These coordinated programs build long-term resilience and reduce outbreak impact through consistent immunization. This collaborative expansion remains a key growth driver for the Ebola Virus Vaccine Market.
Key Trends and Opportunities
Expansion of Single-Dose Immunization Programs
Adoption of single-dose vaccines continues to rise as health agencies focus on fast deployment during outbreak spikes. These programs reduce logistical strain and improve coverage among mobile and high-risk populations. Simplified dosing supports rapid containment efforts in areas with limited medical infrastructure. Manufacturers also invest in improving vector stability, enabling wider use in resource-constrained regions. This expansion stands as a key trend and opportunity in the Ebola Virus Vaccine Market.
- For instance, The Sabin Vaccine Institute began a Phase 2 clinical trial for its Sudan ebolavirus vaccine candidate in Uganda and Kenya in 2024. This trial aimed to recruit approximately 125 volunteers across two initial locations in Uganda and Kenya, with earlier Phase 1b trials (conducted in 2020-2021) having already demonstrated that the single-dose vaccine was safe and immunogenic in adults.
Growing Focus on Cold-Chain Optimization
Advanced cold-chain systems create a strong opportunity to expand vaccine access in remote territories. New portable refrigeration units and heat-stable formulations reduce wastage and improve delivery timelines. Strengthened supply networks enable faster response during emergency surges, helping agencies manage large-scale immunization drives. Investments in energy-efficient storage solutions also support long-term deployment strategies. This enhancement in cold-chain capability remains a key trend and opportunity for the Ebola Virus Vaccine Market.
- For instance, B Medical Systems, which became part of Azenta Life Sciences in 2022, has an installed base of more than half a million medical cold chain products across 170+ countries globally
R&D Shift Toward Multivalent Vaccines
Developers explore multivalent platforms capable of targeting multiple Ebola strains, creating wider protection for populations in endemic zones. These vaccines reduce the need for separate immunization cycles and simplify outbreak management. Progress in vector engineering improves safety, boosts immune response, and accelerates regulatory acceptance. As clinical trials expand, demand for broader-coverage solutions grows among global health agencies. This innovation path forms a major trend and opportunity in the Ebola Virus Vaccine Market.
Key Challenges
Storage and Distribution Barriers
Many regions facing frequent outbreaks lack reliable cold-chain networks, which restricts vaccine distribution. High heat sensitivity increases the risk of dose spoilage, raising operational costs and slowing emergency response. Rural communities often have limited health infrastructure, increasing delays in immunization efforts. Transportation gaps during heavy rainfall or conflict also hinder access. This infrastructure shortfall represents a key challenge in the Ebola Virus Vaccine Market.
Vaccine Hesitancy and Misinformation
Mistrust in vaccination programs reduces acceptance in several endemic communities, driven by misinformation and cultural concerns. Limited health education and fear of side effects weaken public willingness to participate in campaigns. Community resistance slows coverage rates and allows transmission to persist. International agencies must invest in outreach, training, and transparent communication to rebuild trust. This social resistance remains a key challenge in the Ebola Virus Vaccine Market.
Regional Analysis
North America
North America commands the leading share in the Ebola Virus Vaccine Market at approximately 37 % of global revenue, driven by strong biopharma infrastructure and significant government-funded outbreak preparedness. The United States benefits from advanced regulatory frameworks, robust clinical trial capability and comprehensive stockpile programs which support vaccine development and deployment. Premium pricing and proactive demand from health security agencies further reinforce the region’s dominance. As vaccine innovation and strategic procurement remain priorities, North America is likely to sustain its lead throughout the forecast period.
Europe
Europe holds around 20 % share of the Ebola Virus Vaccine Market, benefiting from strong public health institutions, experienced regulatory bodies and coordinated joint procurement mechanisms across the European Union. The region’s emphasis on disease surveillance, cross-border outbreak coordination and inclusion of Ebola in preparedness portfolios drives demand for vaccination programs. Vaccine manufacturers actively engage with European health agencies for clinical validation and supply contracts. With steady investment and heightened awareness of zoonotic threats, Europe remains an important growth region.
Asia Pacific
Asia Pacific accounts for roughly 18 % of the market and is exhibiting the fastest growth rate due to rising focus on infectious-disease preparedness and increased funding in emerging economies. Countries in the region expand their immunization infrastructure, improve cold-chain logistics, and engage in vaccine research collaborations. Growing trade and travel ties with Africa fuel awareness of Ebola risk, prompting regional governments to secure preventive vaccine stockpiles. The combination of large population base and rapidly improving healthcare access presents strong opportunity for market expansion in this region.
Latin America
Latin America contributes approximately 10 % to the global Ebola Virus Vaccine Market. The region benefits from enhanced public-health readiness, regional vaccination initiatives and improved response frameworks following previous regional crises. Health authorities in countries like Brazil and Mexico increasingly support import-and-stockpile strategies for vaccines aimed at haemorrhagic-fever viruses. While growth hurdles remain due to budget constraints and less frequent outbreaks, Latin America nonetheless offers moderate growth potential in line with broader immunisation-capacity improvements.
Middle East & Africa
The Middle East & Africa region holds about 15 % share of the Ebola Virus Vaccine Market, largely due to recurrent outbreaks in African countries, strong interest from international health agencies and vaccine deployment for outbreak containment. Although the healthcare infrastructure in many African nations remains under-resourced, increased donor funding and ring-vaccination campaigns enhance vaccine uptake. The region’s high risk of spill-over events sustains demand for vaccine stockpiling and rapid-response capability. Continued capacity building and improved logistics will accelerate growth here.
Market Segmentations:
By Strain Type
- Vesicular Stomatitis Virus (rVSV)
- Adeno Virus Serotype – 26
By End-use
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Other end-users
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The Ebola Virus Vaccine Market features key players such as Inovio Pharmaceuticals, Inc., Johnson & Johnson, GeoVax Labs, Inc., Mapp Biopharmaceutical, Inc., BioCryst Pharmaceuticals, Inc., GlaxoSmithKline plc, Merck & Co., Inc., Emergent BioSolutions Inc., Immunovaccine Inc. IMV Inc., and Bavarian Nordic A/S. The competitive environment focuses on viral-vector innovation, improved immunogenic profiles and rapid-response vaccine platforms that support emergency deployment. Companies strengthen their positions through clinical trial expansion, regulatory approvals and long-term supply agreements with global health agencies. Manufacturers invest in cold-chain optimization, scalable production capacity and partnerships with international organizations to improve access in high-risk regions. The market also benefits from accelerated research on multivalent vaccines and durable immunity, which helps differentiate product pipelines. Increased emphasis on outbreak preparedness, donor-funded procurement and rapid stockpile distribution continues to shape competitive strategies across the industry, driving sustained innovation and wider global adoption.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- GeoVox Labs, Inc.
- Mapp Biopharmaceutical, Inc.
- BioCryst Pharmaceuticals, Inc.
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Emergent BioSolutions Inc.
- Immunovaccine Inc. IMV Inc.
- Bavarian Nordic A/S
Recent Developments
- In 2023, Bavarian Nordic entered into an agreement to acquire a vaccine portfolio from Emergent BioSolutions, expanding their vaccine pipeline.
- In 2023, GeoVax announced the issuance of a U.S. patent covering their Ebola vaccine platform using a replication-deficient Modified Vaccinia Ankara (MVA) vector expressing ebolavirus antigens.
- In 2023, Inovio Pharmaceuticals, Inc. Presented Phase Ib data showing that INO-4201, a DNA vaccine booster for Merck’s Ebola vaccine Ervebo, produces strong immune responses and is safe and well tolerated.
Report Coverage
The research report offers an in-depth analysis based on Strain Type, End-Use and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will expand as global health agencies increase long-term outbreak preparedness.
- Demand will rise through wider adoption of single-dose vaccines for rapid emergency use.
- Governments will strengthen stockpiling programs to support faster containment responses.
- Multivalent vaccine development will progress to offer broader strain protection.
- Cold-chain improvements will enhance access in remote and high-risk regions.
- Partnerships between manufacturers and international organizations will accelerate large-scale deployment.
- Clinical trials will grow in scope, improving safety, durability and immune-response data.
- Investment in regional manufacturing capacity will reduce reliance on external suppliers.
- Digital surveillance systems will support targeted vaccination strategies and faster outbreak detection.
- Rising awareness of zoonotic threats will sustain long-term market demand.




