1. Preface
1.1. Report Description
1.1.1. Purpose of the Report
1.1.2. Target Audience
1.1.3. USP and Key Offerings
1.2. Research Scope
1.3. Research Methodology
1.3.1. Phase I – Secondary Research
1.3.2. Phase II – Primary Research
1.3.3. Phase III – Expert Panel Review
1.3.4. Approach Adopted
1.3.4.1. Top-Down Approach
1.3.4.2. Bottom-Up Approach
1.3.5. Assumptions
1.4. Market Segmentation
2. Executive Summary
2.1. Market Snapshot: Global Persistent Corneal Epithelial Defects (PCEDs) Market
3. Market Dynamics & Factors Analysis
3.1. Introduction
3.1.1. Global Persistent Corneal Epithelial Defects (PCEDs) Market Value, 2016-2028, (US$ Bn)
3.2. Market Dynamics
3.2.1. Key Growth Trends
3.2.2. Major Industry Challenges
3.2.3. Key Growth Pockets
3.3. Attractive Investment Proposition,2021
3.3.1. Clinical Causes
3.3.2. Product
3.3.3. Application
3.3.4. Distribution Channel
3.4. Porter’s Five Forces Analysis
3.4.1. Threat of New Entrants
3.4.2. Bargaining Power of Buyers/Consumers
3.4.3. Bargaining Power of Suppliers
3.4.4. Threat of Substitute Types
3.4.5. Intensity of Competitive Rivalry
3.5. Value Chain Analysis
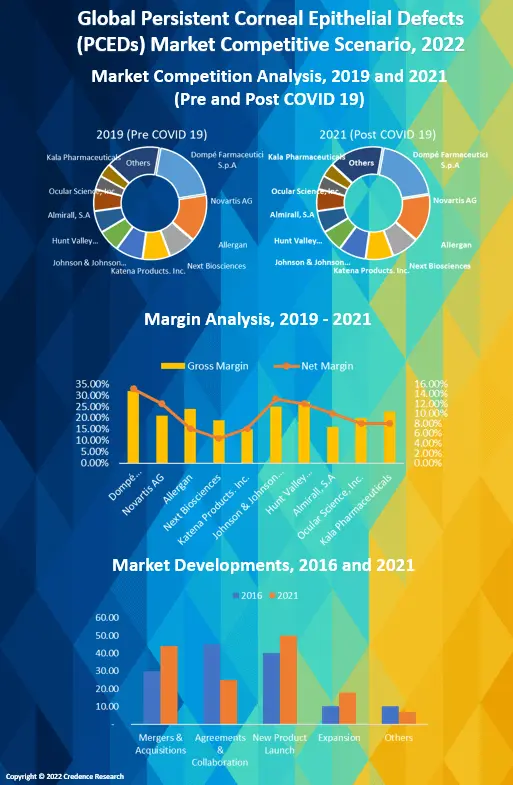
4. Market Positioning of Key Players, 2021
4.1. Company market share of key players, 2021
4.2. Top 6 Players
4.3. Top 3 Players
4.4. Major Strategies Adopted by Key Players
5. COVID 19 Impact Analysis
5.1. Global Persistent Corneal Epithelial Defects (PCEDs) Market Pre Vs Post COVID 19, 2019 – 2028
5.2. Impact on Import & Export
5.3. Impact on Demand & Supply
6. North America
6.1. North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028(US$ Bn)
6.1.1. U.S.
6.1.2. Canada
6.1.3. Mexico
6.2. North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
6.2.1. Overview
6.2.2. Device
6.2.3. Medication
6.3. North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
6.3.1. Overview
6.3.2. Inflammatory Disease
6.3.3. Neurotrophic Keratitis (NK)
6.3.4. Epithelial/Limbal Stem Cell Deficiency
6.3.5. Others
6.4. North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
6.4.1. Overview
6.4.2. Retail Pharmacies
6.4.3. Hospital Pharmacies
6.4.4. Online Pharmacies
6.4.5. Other
7. Europe
7.1. Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028(US$ Bn)
7.1.1. UK
7.1.2. France
7.1.3. Germany
7.1.4. Italy
7.1.5. Russia
7.1.6. Ukraine
7.1.7. Spain
7.1.8. Belgium
7.1.9. Netherland
7.1.10. Austria
7.1.11. Sweden
7.1.12. Poland
7.1.13. Denmark
7.1.14. Switzerland
7.1.15. Rest of Europe
7.2. Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
7.2.1. Overview
7.2.2. Device
7.2.3. Medication
7.2.4. Other
7.3. Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
7.3.1. Overview
7.3.2. Inflammatory Disease
7.3.3. Neurotrophic Keratitis (NK)
7.3.4. Epithelial/Limbal Stem Cell Deficiency
7.3.5. Others
7.4. Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
7.4.1. Overview
7.4.2. Retail Pharmacies
7.4.3. Hospital Pharmacies
7.4.4. Online Pharmacies
7.4.5. Other
8. Asia Pacific
8.1. Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028(US$ Bn)
8.1.1. China
8.1.2. Japan
8.1.3. South Korea
8.1.4. India
8.1.5. Australia
8.1.6. New Zealand
8.1.7. Taiwan
8.1.8. Indonesia
8.1.9. Malaysia
8.1.10. Philippine
8.1.11. Rest of Asia Pacific
8.2. Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
8.2.1. Overview
8.2.2. Device
8.2.3. Medication
8.2.4. Other
8.3. Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
8.3.1. Overview
8.3.2. Inflammatory Disease
8.3.3. Neurotrophic Keratitis (NK)
8.3.4. Epithelial/Limbal Stem Cell Deficiency
8.3.5. Others
8.4. Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
8.4.1. Overview
8.4.2. Retail Pharmacies
8.4.3. Hospital Pharmacies
8.4.4. Online Pharmacies
8.4.5. Other
9. Latin America
9.1. Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028(US$ Bn)
9.1.1. Brazil
9.1.2. Argentina
9.1.3. Peru
9.1.4. Chile
9.1.5. Colombia
9.1.6. Rest of Latin America
9.2. Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
9.2.1. Overview
9.2.2. Device
9.2.3. Medication
9.2.4. Other
9.3. Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
9.3.1. Overview
9.3.2. Inflammatory Disease
9.3.3. Neurotrophic Keratitis (NK)
9.3.4. Epithelial/Limbal Stem Cell Deficiency
9.3.5. Others
9.4. Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
9.4.1. Overview
9.4.2. Retail Pharmacies
9.4.3. Hospital Pharmacies
9.4.4. Online Pharmacies
9.4.5. Other
10. Middle East
10.1. Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028(US$ Bn)
10.1.1. UAE
10.1.2. KSA
10.1.3. Israel
10.1.4. Turkey
10.1.5. Iran
10.1.6. Rest of Middle East
10.2. Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
10.2.1. Overview
10.2.2. Device
10.2.3. Medication
10.2.4. Other
10.3. Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
10.3.1. Overview
10.3.2. Inflammatory Disease
10.3.3. Neurotrophic Keratitis (NK)
10.3.4. Epithelial/Limbal Stem Cell Deficiency
10.3.5. Others
10.4. Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
10.4.1. Overview
10.4.2. Retail Pharmacies
10.4.3. Hospital Pharmacies
10.4.4. Online Pharmacies
10.4.5. Other
11. Africa
11.1. Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028(US$ Bn)
11.1.1. South Africa
11.1.2. Egypt
11.1.3. Nigeria
11.1.4. Rest of Africa
11.2. Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
11.2.1. Overview
11.2.2. Device
11.2.3. Medication
11.2.4. Other
11.3. Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
11.3.1. Overview
11.3.2. Inflammatory Disease
11.3.3. Neurotrophic Keratitis (NK)
11.3.4. Epithelial/Limbal Stem Cell Deficiency
11.3.5. Others
11.4. Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
11.4.1. Overview
11.4.2. Retail Pharmacies
11.4.3. Hospital Pharmacies
11.4.4. Online Pharmacies
11.4.5. Other
12. Global
12.1. Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028(US$ Bn)
12.1.1. Overview
12.1.2. Device
12.1.3. Medication
12.1.4. Other
12.2. Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028(US$ Bn)
12.2.1. Overview
12.2.2. Inflammatory Disease
12.2.3. Neurotrophic Keratitis (NK)
12.2.4. Epithelial/Limbal Stem Cell Deficiency
12.2.5. Others
12.3. Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028(US$ Bn)
12.3.1. Overview
12.3.2. Retail Pharmacies
12.3.3. Hospital Pharmacies
12.3.4. Online Pharmacies
12.3.5. Other
13. Company Profiles
13.1. Dompé Farmaceutici S.p.A
13.2. Novartis AG
13.3. Allergan
13.4. Next Biosciences
13.5. Katena Products. Inc.
13.6. Johnson & Johnson Services, Inc.
13.7. Hunt Valley PharmaLAB
13.8. Almirall, S.A
13.9. Ocular Science, Inc.
13.10. Kala Pharmaceuticals
13.11. Bausch Health
13.12. Integra LifeSciences Corporation
13.13. Laboratoires THEA S.A.S
13.14. Skye Biologics Inc.
13.15. I-MED Pharma inc.
13.16. BioTissue
13.17. Others
List of Figures
FIG. 1 Global Persistent Corneal Epithelial Defects (PCEDs) Market: Research Methodology
FIG. 2 Market Size Estimation – Top Down & Bottom up Approach
FIG. 3 Global Persistent Corneal Epithelial Defects (PCEDs) Market Segmentation
FIG. 4 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2019 (US$ Bn)
FIG. 5 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2019 (US$ Bn)
FIG. 6 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2019 (US$ Bn)
FIG. 7 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2021 (US$ Bn)
FIG. 8 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Geography, 2021 (US$ Bn)
FIG. 9 Attractive Investment Proposition, by Geography, 2021
FIG. 10 Global Market Positioning of Key Persistent Corneal Epithelial Defects (PCEDs) Market Manufacturers, 2019
FIG. 11 Global Persistent Corneal Epithelial Defects (PCEDs) Market Value Contribution, By Type, 2021 & 2028 (Value %)
FIG. 12 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Device, Value, 2016-2028 (US$ Bn)
FIG. 13 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Medication, Value, 2016-2028 (US$ Bn)
FIG. 14 Global Persistent Corneal Epithelial Defects (PCEDs) Market Value Contribution, By Clinical Causes, 2021 & 2028 (Value %)
FIG. 15 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Inflammatory Disease, 2016-2028 (US$ Bn)
FIG. 16 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Neurotrophic Keratitis (NK), Value, 2016-2028 (US$ Bn)
FIG. 17 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Epithelial/Limbal Stem Cell Deficiency, Value, 2016-2028 (US$ Bn)
FIG. 18 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Others, Value, 2016-2028 (US$ Bn)
FIG. 19 Global Persistent Corneal Epithelial Defects (PCEDs) Market Value Contribution, By Distribution Channel, 2021 & 2028 (Value %)
FIG. 20 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Retail Pharmacies, 2016-2028 (US$ Bn)
FIG. 21 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Hospital Pharmacies, 2016-2028 (US$ Bn)
FIG. 22 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Online Pharmacies, 2016-2028 (US$ Bn)
FIG. 23 U.S. Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 24 Rest of North America Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 25 U.K. Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 26 Germany Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 27 France Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 28 Italy Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 29 Spain Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 30 Russia Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 31 BENELUX Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 32 Poland Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 33 Austria Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 34 Rest of Europe Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 35 Japan Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 36 China Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 37 India Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 38 South Korea Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 39 Australia Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 40 Southeast Asia Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 41 Rest of Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 42 Middle East & Africa Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 43 South Africa Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 44 Nigeria Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 45 Egypt Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 46 GCC Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 47 Israel Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 48 Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 49 Mechanicalzil Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 50 Argentina Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 51 Colombia Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 52 Peru Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
FIG. 53 Chile Persistent Corneal Epithelial Defects (PCEDs) Market, 2016-2028 (US$ Bn)
List of Tables
TABLE 1 Market Snapshot: Global Persistent Corneal Epithelial Defects (PCEDs) Market
TABLE 2 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 3 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 4 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (US$ Bn)
TABLE 5 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 6 Global Persistent Corneal Epithelial Defects (PCEDs) Market, by Geography, 2016-2028 (US$ Bn)
TABLE 7 North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 8 North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 9 North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (
TABLE 10 North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 11 North America Persistent Corneal Epithelial Defects (PCEDs) Market, by Country, 2016-2028 (US$ Bn)
TABLE 12 Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 13 Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 14 Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (US$ Bn)
TABLE 15 Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 16 Europe Persistent Corneal Epithelial Defects (PCEDs) Market, by Country/Region, 2016-2028 (US$ Bn)
TABLE 17 Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 18 Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 19 Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (US$ Bn)
TABLE 20 Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 21 Asia Pacific Persistent Corneal Epithelial Defects (PCEDs) Market, by Country/Region, 2016-2028 (US$ Bn)
TABLE 22 Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 23 Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 24 Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (US$ Bn)
TABLE 25 Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 26 Latin America Persistent Corneal Epithelial Defects (PCEDs) Market, by Country/Region, 2016-2028 (US$ Bn)
TABLE 27 Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 28 Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 29 Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (US$ Bn)
TABLE 30 Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 31 Middle East Persistent Corneal Epithelial Defects (PCEDs) Market, by Country/Region, 2016-2028 (US$ Bn)
TABLE 32 Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Type, 2016-2028 (US$ Bn)
TABLE 33 Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Application, 2016-2028 (US$ Bn)
TABLE 34 Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Clinical Causes, 2016-2028 (US$ Bn)
TABLE 35 Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Distribution Channel, 2016-2028 (US$ Bn)
TABLE 36 Africa Persistent Corneal Epithelial Defects (PCEDs) Market, by Country/Region, 2016-2028 (US$ Bn)